Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 09 November 2023

Understanding nucleic acid sensing and its therapeutic applications
- Ling-Zu Kong 1 , 2 ,
- Seok-Min Kim 1 ,
- Chunli Wang 1 ,
- Soo Yun Lee 1 ,
- Se-Chan Oh 1 ,
- Sunyoung Lee 1 , 3 ,
- Seona Jo 1 , 4 &
- Tae-Don Kim ORCID: orcid.org/0000-0002-5910-4264 1 , 4 , 5 , 6
Experimental & Molecular Medicine volume 55 , pages 2320–2331 ( 2023 ) Cite this article
6854 Accesses
16 Citations
3 Altmetric
Metrics details
- Immunotherapy
- Innate immunity
Nucleic acid sensing is involved in viral infections, immune response-related diseases, and therapeutics. Based on the composition of nucleic acids, nucleic acid sensors are defined as DNA or RNA sensors. Pathogen-associated nucleic acids are recognized by membrane-bound and intracellular receptors, known as pattern recognition receptors (PRRs), which induce innate immune-mediated antiviral responses. PRR activation is tightly regulated to eliminate infections and prevent abnormal or excessive immune responses. Nucleic acid sensing is an essential mechanism in tumor immunotherapy and gene therapies that target cancer and infectious diseases through genetically engineered immune cells or therapeutic nucleic acids. Nucleic acid sensing supports immune cells in priming desirable immune responses during tumor treatment. Recent studies have shown that nucleic acid sensing affects the efficiency of gene therapy by inhibiting translation. Suppression of innate immunity induced by nucleic acid sensing through small-molecule inhibitors, virus-derived proteins, and chemical modifications offers a potential therapeutic strategy. Herein, we review the mechanisms and regulation of nucleic acid sensing, specifically covering recent advances. Furthermore, we summarize and discuss recent research progress regarding the different effects of nucleic acid sensing on therapeutic efficacy. This study provides insights for the application of nucleic acid sensing in therapy.
Similar content being viewed by others
DNA-sensing pathways in health, autoinflammatory and autoimmune diseases
Heteromultivalency enables enhanced detection of nucleic acid mutations
Cytosolic DNA sensing by cGAS: regulation, function, and human diseases
Introduction.
As a complementary host defense mechanism, the vertebrate immune system consists of both innate and adaptive immune responses 1 . Although innate immunity cannot confer specificity for host defense or form immune memory, its defense mechanisms can recognize and destroy most microbes within minutes to hours. Cells detect external components of pathogens, or pathogen-associated molecular patterns (PAMPs), through pattern recognition receptors (PRRs), which largely comprise Toll-like receptors (TLRs) and C-type lectin receptors (CLRs). In addition, endosomal TLRs and cytoplasmic nucleic acid receptors, including nucleotide-binding and oligomerization domain NOD-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs), and cyclic GMP-AMP synthase (cGAS), recognize cell-invading exogenous nucleic acids; adaptive immune cells are among those that can detect exogenous pathogens via these receptors.
Nucleic acids, which are the genetic building blocks of all organisms, are potent PAMPs released during viral infection and are discerned as exogenous nucleic acids by specialized PRRs. Based on their different forms of nucleic acids, pathogen-derived double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and DNA are recognized by TLR3, TLR7/8, and TLR9, respectively, in human endosomes. In contrast, nucleic acid (NA)-sensing mechanisms in the cytoplasm contribute to immunity mainly by recognizing invading RNA by RLRs and invading DNA by cGAS and interferon gamma-inducible 16 (IFI16). Activated NA-sensing PRRs transduce signals to aptamer molecules or directly recruit downstream proteins that mediate cytokine and type I and III interferon (IFN) production by activating nuclear factor (NF)-κB and interferon regulatory factor (IRF) proteins, respectively 2 .
Nucleic acid sensors not only mediate immune defense against pathogens but also detect tumor-derived DNA to trigger antitumor immune responses. Therefore, nucleic acid receptors are potential targets for cancer therapy 3 , 4 . Organismal development and aging are accompanied by apoptosis, through which nucleic acids are released from cells 5 . Many inflammatory and autoimmune diseases are associated with the dysfunctional or abnormal activation of nucleic acid receptors, which are considered attractive targets for the development of therapeutic agonists or antagonists 6 . To maintain homeostasis and induce optimal immune responses, multiple mechanisms regulate the NA-sensing factors that distinguish between self- and non-self-derived nucleic acids 7 , 8 . Nucleic acid sensors have exhibited considerable potential in immunotherapy and the treatment of autoimmune diseases; however, the mechanisms underlying their modulatory roles are unclear. For a long time, innate immune-activating molecules were used as adjuvants in vaccines 9 ; however, the immunogenicity of mRNA has been found to be the main factor diminishing the efficiency of mRNA vaccines 10 . Therefore, greater attention is being paid to the development of nucleic acid vaccines that do not activate innate immunity and produce more antigenic proteins. In this review, we discuss recent advances in understanding the mechanisms and regulation of NA-sensing and related signaling in different treatments. To highlight the clinical implications of NA-sensing mechanisms, we outline some ways of evading NA-sensing pathways during therapy.
Nucleic acid sensing in endosomes
TLRs constitute a class of transmembrane innate immune receptors that are evolutionarily conserved and induce immune responses by recognizing distinctive PAMPs. TLRs are single-transmembrane proteins composed of an extracellular N-terminal domain, which recognizes ligands; a transmembrane domain; and a cytoplasmic Toll/interleukin 1 receptor (TIR) domain. Human NA-sensing TLRs include TLR3, TLR7, TLR8, and TLR9, which localize to the intracellular compartment membranes and recognize viral and bacterial cytosolic components, such as nonmethylated CpG DNA and single- and double-stranded RNA.
Structure and ligands of nucleic acid-sensing TLRs
TLR3, the first described viral TLR, recognizes dsRNAs larger than 40 bp, which are released during RNA virus replication 11 . TLR3-induced responses increase in intensity with increasing dsRNA sequence length; however, the underlying molecular mechanism underlying this increase remains unclear 12 . Two monomeric forms of TLR in solution bind to the dsRNA ligand to form a dimer; the dimerized TLR3 clamps around the dsRNA without any detectable sequence affinity specificity 13 . In contrast to other NA-sensing TLRs, TLR3 is expressed in immune cells as well as some nonimmune cells, such as neurons and keratinocytes 14 , and its widespread expression enables it to play a crucial role in RNA virus infection.
TLR7 and TLR8 specifically recognize ssRNA in endosomes. TLR7 and TLR8 preferentially bind guanosine and uridine, respectively, but contain other ssRNA-binding sites 15 . TLR9 recognizes ssDNA containing unmethylated CpG sequences (commonly found in bacteria and viruses). TLR9 harbors two DNA-binding sites—CpG- and 5′-xCx-binding sites 16 . RNA:DNA hybrids are also recognized by TLR9 17 .
Trafficking and activation of nucleic acid-sensing TLRs
All NA-sensing TLRs are synthesized in the endoplasmic reticulum (ER) and transported to endosomes via the canonical secretory pathway 18 . However, the characteristics of the transport routes and compartments in which they ultimately reside are surprisingly diverse 19 . Unc93B1, an ER multiple transmembrane protein, is an essential trafficking molecule for all NA-sensing TLR proteins 20 that mediates the differential transport of TLRs 21 . Inactive TLR9 is native to the ER of dendritic cells (DCs) and B cells, from which it is transported first to the cytoplasmic membrane and then internalized into endosomes via adaptor protein 2 (AP2)-mediated endocytosis 22 , whereas TLR7 recruits AP4 directly for subsequent translocation to endosomes 22 (Fig. 1 ). TLRs in endosomes undergo proteolytic cleavage, thereby producing functional receptors that interact with nucleic acid ligands 23 .
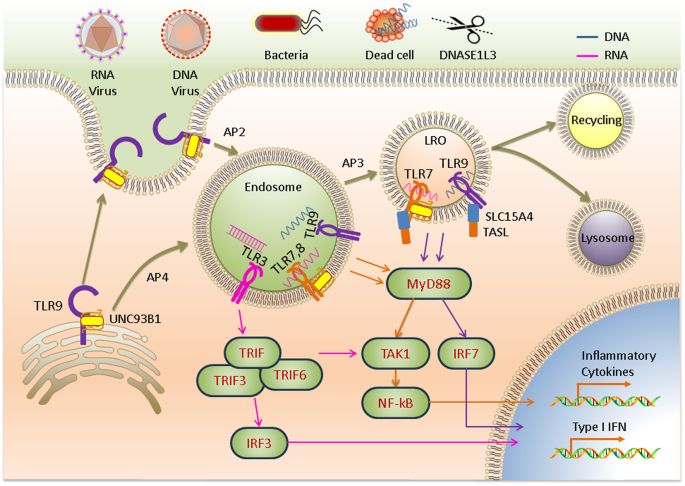
TLR9 is first trafficked to the plasma membrane and then internalized into the endosome via AP2, where TRIF is recruited to activate downstream transcription factors. TLR7 and TLR9 depend on AP4 for localization to the endosome to activate the TAK1 signaling pathway via the recruitment of MyD88. AP3 further mediates TLR localization to lysosome-related organelles (LROs), where type I IFN gene activation is mediated.
Activation of all NA-sensing TLRs is restricted to endosomes 19 . This recognition pattern allows cells to recognize and sequester pathogens in the endosomal compartment without risking infection, and the contents are subsequently sorted for degradation or recycling in a small GTPase-dependent manner. Pathogens enter an endosome via endocytosis. After binding to nucleic acids, a TLR forms a complex, either it’s a hetero or homodimer, and the intracellular TIR domains of the dimerized TLRs come into close contact with each other to activate downstream signal transduction. The signaling cascade depends on the types of ligands, interacting TLRs, and downstream bridging molecules. TLR3 homodimers directly recruit TRIF in response to viral dsRNA binding. Other NA-sensing TLRs trigger the NF-κB and/or IRF signaling pathways via MyD88 to induce cytokine and type I IFN production, promoting inflammatory and antiviral responses, respectively 24 . TIR domain-containing adaptor-inducing interferon-β (TRIF), TNF-receptor-associated factor 3 (TRAF3), and TRAF6 form a complex that activates IRF3 signaling to produce type I IFN 21 . However, TLR7 and TLR9 are dependent on AP3-based transport from the endosome to lysosome-related organelles, which is regulated by the peptide transporter protein solute carrier family 15 member 4 (SLC15A4) in the endosomal compartment 25 . TLR7, TLR8, and TLR9 interact with the TLR adaptor TASL in a lysosomal SLC15A4-dependent manner and activate IRF signaling to produce type I IFN 14 , 26 (Fig. 1 ).
Because nucleic acids can be derived from various sources, the regulation of TLR ligand availability is essential to balance the pathogen-sensing and self-recognition abilities of TLRs and modulate inflammatory responses, which primarily involve ligand internalization, nucleic acid digestion or processing, and the cytoplasmic transport of ligands.
Regulation of nucleic acid-sensing TLRs
Nucleic acid digestion by nucleases regulates ligand availability. Generally, ligand digestion in the endosomal compartment negatively regulates TLR responses, preventing the generation of autoimmune responses and the excessive activation of antiviral innate immune responses. Nucleases that play a regulatory role in the activation of TLRs include ribonuclease (RNase) T2, deoxyribonuclease (DNase) I-like 3 (DNASE1L3), DNase II, phospholipase D3 (PLD3), and PLD4 14 . RNase T2 is widely expressed in a variety of cell types and negatively regulates TLR3 activation by degrading RNA in endosomal compartments; moreover, it is required for the activation of TLR7 and TLR8 27 , 28 . RNase T2 deficiency or mutations can cause cystic leukoencephalopathy 29 . The endonuclease DNase I-like 3 is expressed in innate immune cells and degrades nucleic acids carried by dead cells before it is internalized 30 (Fig. 1 ). DNASE1L3 possesses a unique positively charged and highly hydrophobic C-terminal domain (CTD) that allows it to digest DNA bound to proteins or lipids, which likely contributes to cell transfection difficulties 31 . Functional mutations in the DNASE1L3 gene cause a rare form of pediatric systemic lupus erythematosus (SLE) 32 . DNase II degrades DNA in the endosomal compartment, while loss-of-function mutations in DNASE2 cause type I interferonopathies 33 . PLD3 and PLD4 degrade TLR7 and TLR9 ligands in endolysosomes. Mice deficient in PLD3 and PLD4 suffer from fatal diseases during the early stages of life 34 . Nuclease deficiency can cause large amounts of nucleic acids to enter the cytoplasm during subsequent endosome rupture, activating the cytosolic NA-sensing pathway and causing type I interferonopathies, which can be alleviated by eliminating type I IFN or blocking TLR trafficking 35 . The physiological characteristics of some nucleases that play partial roles or are functionally redundant, such as RNase A and DNase I, require further study 36 .
The amount of ligand internalized by cells is another critical factor affecting ligand availability. It has been shown that the uptake of extracellular immune complexes containing self-nucleic acids is associated with receptor for advanced glycosylation end products (RAGE) 37 . Self-nucleic acids interact with the antimicrobial peptide LL37 or HMGB1 to promote endosomal uptake of nucleic acids and reduce nuclease degradation, which in turn stimulates the activation of NA-sensing TLRs via self-nucleic acids 38 , 39 . The transport of ligands from the nuclear endosome to the cytoplasm can reduce the concentration of ligands in the endosome. SIDT1 and SIDT2 can promote dsRNA escape from the endosome into the cytoplasm and activate antiviral immune signaling 40 , 41 .
RNA sensing in the cytosol
Notably, NA-sensing TLRs are mostly expressed in immune cells. However, epithelial cells and fibroblasts on the mucosal surface, which are exposed to the external environment and are susceptible to infection, can still produce an effective innate immune response to prevent pathogen proliferation 42 . Different cell types employ different nucleic acid recognition mechanisms to combat viral invasion 43 . Various cytoplasmic RNA-sensing mechanisms have been identified.
Structure and ligands of RLRs
RLRs have been extensively studied as primary cytoplasmic RNA-monitoring mechanisms. RLRs constitute a class of cytoplasmic RNA helicases that detect viral RNA accumulated during infection or replication in a nonsequence-specific manner and elicit antiviral immune responses through the production of type I IFN 44 , 45 . In contrast to TLRs, RLRs are expressed by most cell types. The RLR family includes RIG-I, melanoma differentiation-associated gene-5 (MDA-5), and laboratory of genetics and physiology 2 (LGP2). All RLRs have conserved structural domains and contain a central DExD/H-box helicase and CTD. RIG-I and MDA5 also carry two N-terminal caspase recruitment domains (CARDs) that are primarily responsible for signal transduction. In the inactivated state of RIG-I, the CARDs interact with the helicase domain to maintain an autoinhibited conformation. Downstream signaling is initiated by exposure to CARDs when RNA binds to the helicase domain and CTD. This conformational change is thought to be triggered by a V-shaped pincer domain consisting of a unique elbow-shaped helical extension of the CTD with the HEL2 helicase domain 46 .
RIG-I recognizes the 5′-ppp structure of an RNA and the blunt base-paired 5′ end. DsRNA are characterized by these ligand structures. Some RNA secondary structures consist of the genetic material of many RNA viruses that are generally not found in healthy host cells. In addition, RIG-I can be induced to produce a weaker signal by RNA without the 5′-PPP structure 47 . Some differences between RLRs have been described 48 . RIG-I recognizes relatively short dsRNAs, while the ligand preferences of MDA5 have not be fully elucidated; however, it is generally believed that MDA5 preferentially binds to long dsRNAs (>1 kb) 49 . The open C-shaped structure of MDA5 confers the ability to assemble filamentous oligomers along long dsRNAs 50 . LGP2 can bind dsRNA; however, it is thought to regulate RLRs because it lacks an NA-sensing signaling function.
Activation of RLRs
RLRs exposed to CARDs are fully activated by the action of various enzymes and subsequently depend on interactions with 14-3-3ε 51 and 14-3-3η 52 , which are members of the 14-3-3 protein family, to mediate the relocalization of RIG-I and MDA5, respectively, to mitochondria. Mitochondrial antiviral-signaling (MAVS) protein is a common adapter protein associated with RIG-I and MDA5 and is localized to the inner mitochondrial membrane. RLRs interact with the homologous CARD of MAVS and subsequently induce TRAF-binding motifs to recruit TRAF2, TRAF5, TRAF6, and TRADD, which mediate the activation of IRF3 or IRF7 via the action of the cytoplasmic kinase TANK-binding kinase 1 (TBK1) to produce type I and III IFNs 15 . In addition, MAVS signaling mediates the stimulation of proinflammatory cytokines through the induction of NF-κB activation via the IKK complex 53 (Fig. 2a ).
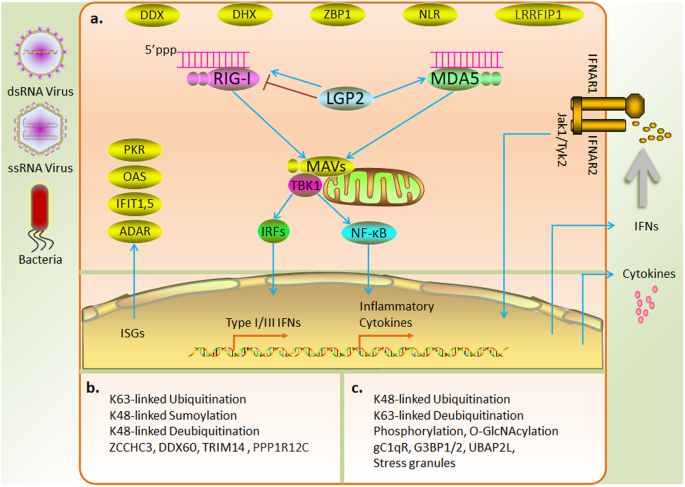
a RLRs are activated by RNA derived from a virus or bacteria and mediate the production of type I and III IFNs and inflammatory cytokines via the MAVS adaptor protein. Interferons released into the extracellular compartment activate interferon-stimulated genes and induce direct antiviral responses. b Positive regulation of RLRs by posttranslational modifications and interacting proteins. c Negative regulation of RLRs by posttranslational modifications and interacting proteins.
LGP2, which lacks a signaling structural domain, has been shown to regulate RIG-I and MDA5 in several studies. LGP2 inhibits RIG-I activation through ligand competition 54 or by directly impeding the oligomerization and signal activation of RIG-I, which is mediated through the RIG-I CTD domain 55 . In addition, LGP2 interacts with tripartite motif-containing 25 (TRIM25) to inhibit RIG-I ubiquitination 56 . In contrast, LGP2 facilitates MDA5 signaling 57 , 58 . During viral infection, LGP2 has also shown to promote both RIG1 and MDA5 signaling 59 (Fig. 2a ). In conclusion, the characterization of the regulatory role of LGP2 under specific physiological conditions requires further study.
Regulation of RLRs
In addition to LGP2, multiple intracellular mechanisms participate in the regulation of RLR activity, including multiple posttranslational modifications (PTMs) and protein interactions. Ubiquitination of RIG-I CARD via K63 linkages, mediated by the ubiquitinated proteins TRIM25, Riplet, TRIM4, and Mex-3 RNA-binding family member C (Mex3c), promotes RIG-I oligomerization and signal transduction 60 , 61 , 62 , 63 ; in contrast, polyubiquitination via K48 linkages, mediated by ring finger protein 122 (RNF122), RNF125, Casitas B-lineage lymphoma (c-Cbl), and TRIM40 64 , induces RIG-I degradation. Deubiquitinases, including ubiquitin-specific peptidase 3 (USP3), USP21, and CYLD lysine 63 deubiquitinase (CYLD), attenuate the antiviral response by removing the K63-linked polyubiquitin chain. In contrast, USP4 and USP15 enhance the stability of RIG-I by hydrolyzing the K48-linked ubiquitin chain and exerting a positive regulatory effect 65 . SUMOylation prevents RLR degradation via K48-polyubiquitin-dependent degradation, thereby stabilizing RLR in the early stages of viral infection 66 . Additionally, phosphorylation causes RIG-I to be autoinhibited 67 . A recent study showed that O-GlcNAcylation inhibited RIG-1 signaling by modifying MAVS 68 (Fig. 2b, c ). Although the PTMs related to MDA5 signaling have been studied relatively rarely, it is likely that PTMs regulate MDA5 in a manner similar to their regulation of RIG-I.
Many dsRNA-binding proteins participate in the regulation of RLRs. PACT positively regulates RLRs by interacting with the CTD of RIG-I or promoting MDA5 oligomerization 69 , 70 . The zinc finger protein ZCCHC3 has recently been shown to function as a coreceptor for RIG-I and MDA5 71 . DExD/H-box helicase 60 (DDX60) promotes RIG-I-dependent innate immune responses 72 . In addition to covalent modifications, TRIM14 enhanced RIG-I signaling by recruiting NF-κB essential regulator (NEMO) to the MAVS complex via the ubiquitin chain 73 . A recent study demonstrated that PPP1R12C relocalization triggered by viral infection or RNA delivery reagents promoted downstream signaling by mediating the dephosphorylation of RLRs 74 . In contrast, the complement component C1q (gC1qR) on mitochondria inhibited RIG-I- and MDA5-dependent antiviral responses 75 . Stress granules formed by the aggregation of the key nucleating factors G3BP1/2 and UBAP2L with stalled ribosome–mRNA complexes inhibited excessive activation of RLR signaling and prevented viral replication through unknown physiological functions 76 (Fig. 2b, c ).
Other RNA sensors
Several other cytoplasmic RNA sensors trigger antiviral responses via transcription factors, including certain DExD/H-box RNA helicases (which recognize RNA through their conserved motifs and are involved in the activation of TLR and RLR downstream signaling pathways), NLRs (which induce inflammasome activation by binding RNA), the LRR domain of flightless-1-interacting protein 1 (LRRFIP1, which binds dsRNA and dsDNA to induce type I IFN production through β-catenin phosphorylation), Z-DNA binding protein (ZBP1 77 , which induces activation of innate immunity and PANoptosis through recognition of Z-DNA and Z-RNA) (Fig. 2a ), and HMGB (which may act as a cosensor for various PRRs) (see reviews 47 , 78 ).
Various RNA sensors with direct antiviral activity are expressed in cells; these sensors include 2′,5′-oligoadenylate synthetase (OAS), RNA-regulated protein kinase (PKR), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), and adenosine deaminase acting on RNA (ADAR), the expression of which depend on type I IFN or PRR signaling 7 , 79 (Fig. 2a ). OAS binds dsRNA and catalyzes the generation of 2′-5′-linked oligoadenylates (2–5A) from substrate ATP to degrade virus-derived dsRNA by mediating the activation of RNase L. PKR can be activated by viral-derived dsRNA or short 5′-ppp RNA-containing secondary structures. Activated PKR mediates the phosphorylation of the α-subunit of eukaryotic initiation factor 2 (eIF2) to inhibit translation initiation. IFIT1 binds to ssRNAs containing the 5′-ppp terminus to repress cap-dependent RNA translation. ADAR-edited cell-derived self-RNAs can evade NA-sensors; however, A-I editing may lead to amino acid substitutions and loss of function of viral proteins 80 .
DNA sensing in the cytosol
Cells infected with a DNA virus but that do not express TLR9 produce high levels of type I IFN 81 . Therefore, ZBP1 82 and RNA Pol III 83 were initially identified as cytoplasmic DNA sensors. However, subsequent studies revealed that RNA Pol III-mediated innate immune responses were dependent on poly (dA:dT)-converted RNA ligands with 5′-triphosphate and double-stranded secondary structures to activate the RIG-I/MAVS pathway (Fig. 3a ), and interferon production was induced in mouse cells lacking MAVS. Similarly, ZBP1 plays a role only in specific cell types, suggesting that DNA activates unknown DNA-sensing pathways in the cytoplasm in a nonsequence-specific manner.
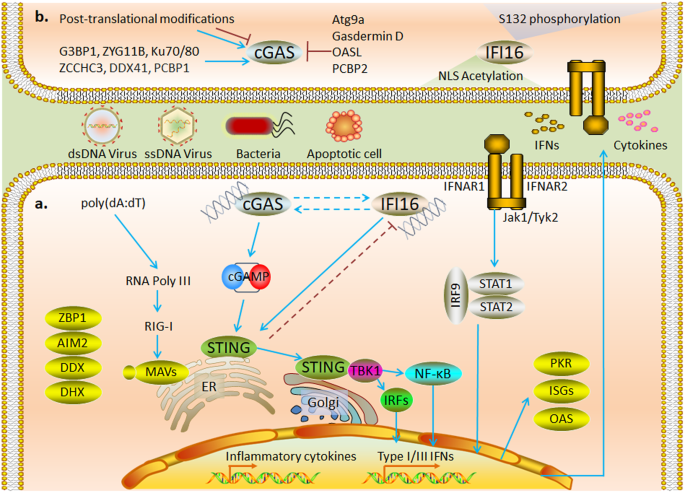
a cGAS and IFI16, as major DNA receptors in the cytoplasm, induce STING-dependent inflammatory cytokines and IFN production and inhibit viral replication by activating interferon-stimulated genes. b Posttranslational modifications of amino acid residues at different sites regulate the activity of cGAS and nuclear localization of IFI16; a variety of proteins have been shown to participate in regulating the activity of cGAS.
Structure and ligands of cytosolic DNA sensors
Interferon-gamma inducible protein 16 (IFI16) and cGAS have been identified as cytoplasmic DNA receptors. Mammalian cGAS belongs to the cGAS/DncV-like nucleotidyltransferase (CD-NTase) family, the members of which are structurally similar to OAS 84 . cGAS contains a disordered N-terminus that anchors its inactivated form to the inner cell membrane 85 , a central NTase domain, and a C-terminal Mab-21 homology domain containing the zinc-ribbon/thumb motif. cGAS binds to dsDNA to form a dimer, followed by DNA sequestration via liquid-phase condensation 86 . cGAS–DNA condensation protects the DNA against Trex1 nuclease-mediated DNA degradation 87 . cGAS activation by dsDNA is DNA length dependent 88 , as more than 45 bp of a dsDNA molecule binding to the A and B sites of each hcGAS molecule and with a third binding site that promotes the stability of the complex. 89 . In addition, cGAS generates innate immunity by recognizing RNA:DNA hybrid molecules generated by intracellular reverse transcription of the HIV-1 virus 90 . PQBP1 acts as an intracellular receptor by which HIV cDNA is recognized by cGAS 91 .
IFI16 (p204 in mice), a member of the ALR family, contains a pyrin structural domain (PYD) and two DNA-binding hematopoietic interferon-inducible nuclear antigens with 200-amino-acid repeat (HIN) structural domains. IFI16 also binds to dsDNA in a length-dependent manner 92 . When binding dsDNA molecules, the PYD structural domain of IFI16 assembles into filamentous oligomers in synergistic association with neighboring PYDs and induces STING-dependent type I IFN production 93 . In addition, IFI16 recognizes viral RNA, promotes RIG-I activation through direct interaction, and upregulates RIG-I transcription by recruiting RNA polymerase II, which provides evidence of crosstalk between RNA- and DNA-sensing mechanisms 94 .
Activation of cytosolic DNA sensors
Activation of cGAS requires nuclear export signals to mediate its cytoplasmic localization 95 . cGAS catalyzes the generation of the second messenger cGAMP from ATP and GTP, induces IFN production through activation of the STING-TBK1-IRF3 axis, and mediates cytokine production through activation of NF-κB. IFI16 shuttles between the nucleus and cytoplasm and mediates interferon production via a STING-dependent cytosolic signaling pathway 92 , 96 (Fig. 3a ). In a sequencing analysis of four cell types, IFI16 was found to exert a crucial effect on the transfection efficiency of plasmid DNA (pDNA) 97 .
STING is predominantly located on the ER outer membrane and is expressed in most cells. STING mediates the cytoplasmic dsDNA-induced antiviral innate immune response as an adaptor molecule in response to cGAS and IFI16. cGAMP directly binds to STING, induces STING movement from the ER to the Golgi apparatus, and ultimately recruits TBK1 to colocalize with STING puncta in the perinuclear region. TBK1 recruitment is critical for STING-mediated IRF3 and NF-κB activation 98 . DNA-bound IFI16 interacts with STING in the cytoplasm to recruit and activate TBK1-IRF3 signaling and mediate IFN production 99 (Fig. 3a ).
Regulation of cytosolic DNA sensors
cGAS is strictly regulated to produce a balanced immune response 100 . Intracellular nucleases are essential for ligand availability in cytoplasmic DNA sensors. Deficiency or mutation in TREX1, RNASEH2, or SAMHD1 leads to cGAS-dependent type I IFN production 101 , 102 , 103 . In addition, multiple mechanisms participate in the regulation of the posttranslational modifications of cGAS 104 . Elimination of the K48-linked ubiquitinated chain suppresses P62-mediated autophagic degradation of cGAS 105 . However, the abrogation of K63-linked polyubiquitination promotes the DNA-binding ability of cGAS 106 . Interestingly, the deubiquitinating enzyme OTUD3 promotes cGAS-mediated DNA sensing but inhibits RLR-mediated RNA sensing 107 . The acetylation of lysine residues in the unstructured N-terminal region of hcGAS promotes its activation 108 . In contrast, acetylation of Lys384/Lys394/Lys414 inhibited cGAS activation 109 . SUMOylation at different sites exerts different regulatory effects on cGAS. SENP2-mediated deSUMOylation induces cGAS degradation during late viral infection 110 . However, SENP7-mediated deSUMOylation enhances cGAS activation 111 . AKT, CDK1, DNA-PK, and Aurora A-mediated phosphorylation of hcGAS can inhibit its enzymatic activity 112 , 113 , 114 . O-GlcNAcylation has been reported to regulate NA-sensing in various cells, although the mechanism remains unclear 115 . OGT has recently been found to activate cGAS-mediated innate immune responses by enhancing the stability of SAMHD1, thereby promoting intracellular dNTP depletion and generating DNA replication intermediates 116 (Fig. 3b ).
High acetylation and phosphorylation rates of endogenous IFI16 have been found in lymphocytes and are mainly associated with nuclear localization. IFI16 carries a nuclear localization signal (NLS) at the N-terminus, and the NLS motif is modified by acetyltransferase p300 to promote accumulation in the cytoplasm 117 . In contrast, phosphorylation by CD2 on S132 promotes the nuclear localization of IFI16 118 . Recent studies revealed that clearly localized IFI16 prevented DNA viral invasion via its effect on different pathways 119 (Fig. 3b ).
Several proteins have been found to mediate cGAS signaling by interacting with ligands or regulating ligand action. Among these proteins, G3BP1, ZYG11B, Ku, and ZCCHC3 promote cGAS-mediated innate immune responses by facilitating DNA binding and condensation 120 , 121 , 122 , 123 . DEAD-box helicase 41 (DDX41) promotes cGAS activation by regulating DNA stabilization via its helicase activity 124 . Others, such as Atg9a and Gasdermin D, inhibit STING-dependent innate immune responses by mediating autophagy 125 , 126 . OASL suppresses IFN production by specifically binding to cGAS during DNA virus infection 127 . Notably, poly(rC)-binding protein 1 (PCBP1) facilitates the binding of cGAS to DNA, whereas PCBP2 interacts with cGAS and prevents its excessive activation 128 , 129 (Fig. 3b ). Additionally, cGAS, IFI16, and STING regulate each other. cGAS may contribute to the innate immune response by increasing the stability of IFI16 130 , and IFI16-mediated TBK1 recruitment is essential for cGAMP-mediated STING activation 96 , 131 . STING negatively regulates antiviral immune responses through TRIM21-mediated ubiquitinated degradation of IFI16 132 . Moreover, the transport of extracellular second messenger cyclic dinucleotides (CDNs) by SLC19A1 133 , SLC19A2 134 , LRRC8 135 , LL37 136 , P2X7R 137 , and Connexin 138 is essential for the activation of intracellular STING. ABCC1 has recently been identified as a cGAMP export protein 139 . ENPP1 attenuates STING activation in bystander cells by degrading extracellular cGAMP 140 .
Other cytosolic DNA sensors
Other DNA sensors can recognize DNA in specific cell types or may recognize only specific sequences, mainly, the DExD/H-box helicases DHX9 and DHX36 (which recognize CpG DNA to activate the TLR downstream signaling pathway), DDX41 and DDX60 (which enhance the type I IFN response by binding dsDNA), and AIM2 (which triggers the inflammasome pathway by binding dsDNA to produce IL-1β and IL-18) (see reviews 42 , 141 ) (Fig. 3a ).
Nucleic acid sensing as a promising therapeutic target
NA-sensing exerts both pro- and antitumor effects at different stages of tumorigenesis. Genomic instability typically produces autoimmunogenic DNA in cancer cells. Therefore, NA-sensing-mediated IFN production contributes to DC maturation and tumor-specific T-cell responses 142 . However, in vitro studies have revealed that NA-sensing pathways in several cancer cells are inhibited by JAK2-STAT3-mediated signaling 143 . External activation of NA-sensing has shown enhanced antitumor effects in a variety of cancers. However, in metastatic cancer, the cGAS-STING-TBK1 axis-mediated inflammatory response is positively associated with tumor metastasis. These opposing effects may be associated with the type and stage of the tumor 8 .
NA-sensing-associated mechanisms also play different regulatory roles in gene therapy. Genetic vaccines, including DNA and RNA vaccines consisting the nucleic acids of target genes, are injected directly into the body to induce innate and adaptive immune responses 144 , 145 . pDNA is an intrinsic adjuvant for DNA vaccines and is essential for the activation of resident antigen-presenting cells through activation of the innate immune response via the action of STING-TBK1 146 , 147 . However, type I IFN produced by activated nucleic acid induction inhibits the translation of mRNA vaccine-encoded antigenic proteins, thereby reducing antigen-specific immunity 148 , 149 . Type I IFN is probably critical for enhancing the early immune response but is also the main cause of side effects 150 . For optimal treatment outcomes, it is essential that the immunostimulation and transfection efficiency of nucleic acids be balanced when designing therapeutic strategies 151 .
Positive regulation of nucleic acid sensing in therapy
The activation of NA-sensing in cancer cells promotes hot tumor transformation through the production of type I IFN and cytokines 152 . Type I interferons also upregulate the expression of major histocompatibility complex (MHC) class I molecules in antigen-presenting cells, which present processed cancer cell-derived antigen molecules to CD8+ T cells 152 . Stimulating the production of type I and III IFNs in CD4+ T cells confers self-protection against HIV infection and enhances the ability of CAR-T cells to clear tumor cells 153 . In addition, cGAS-mediated cGAMP release from cancer cells activates adjacent immune cells 154 , 155 . Studies have shown that the DNA released from tumor cells after chemotherapy and radiotherapy activates NA-sensing signals that synergistically enhance antitumor effects 156 , 157 , 158 . Agonists of cGAS, STING, and RIG-I potentiate the antitumor activity of immune cells 159 , 160 , 161 ; for example, the combination of a STING agonist and a PD1 blocker showed therapeutic effects in tumors with low immunogenicity 162 . Similarly, the innate immune response mediated by RIG-I ligands in combination with CTLA-4 blockade enhanced adaptive immune response-mediated antitumor effects 163 . Furthermore, this combination therapy can enhance the antitumor effect of the anti-PD1 antibody in a cGAS-dependent manner by inhibiting the protein arginine methyltransferase PRMT1- and PRMT5-mediated methylation of the cGAS residues Arg133 and Arg124, respectively 164 , 165 (Fig. 4a ). A recent study suggested that inducing RIG-I-dependent OAS/RNase L-mediated apoptosis is a potential strategy for cancer immunotherapy 166 .
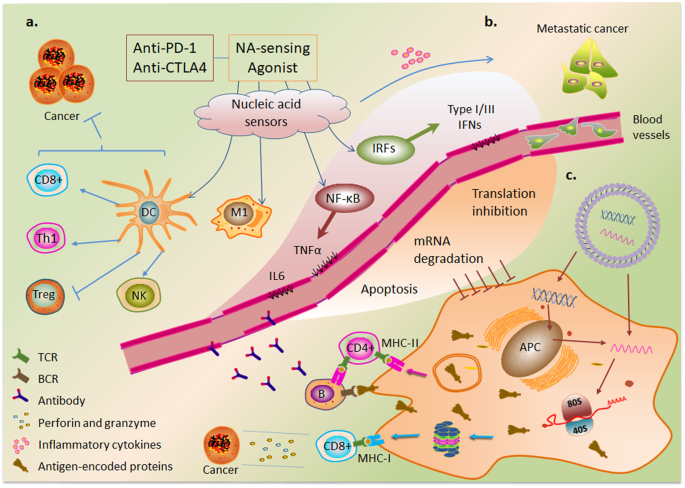
a NA-sensing promotes the antitumor therapeutic efficacy of immune checkpoint inhibitors by inducing dendritic cell (DC) maturation and tumor-specific T-cell responses and promotes the differentiation of macrophages into M1 proinflammatory macrophages. b In the metastatic stage of cancer, NA-sensing-induced inflammatory cytokines exhibit cancer-promoting effects. c Model of antigen-specific immunity mediated by nonviral gene therapies and the negative regulatory effects of NA-sensing on therapeutic transgenes.
Because the basic components of pDNA, such as the TLR9 agonist, are immunogenic, the unmethylated CpG sequence is commonly used as a vaccine adjuvant 167 . CpG oligodeoxynucleotides (ODNs) stimulate the maturation and survival of plasmacytoid DCs and accelerate regulatory T (Treg) cell differentiation and depletion through the activation of TLR9 168 , 169 . Recent studies revealed that SARS CoV-2 mRNA vaccination exposes HIV to CD8+ T cells 170 . A small-molecule agonist of RIG-I, KIN1148, exhibits an adjuvant effect on influenza virus vaccine immunity 171 . The dsRNA analog poly(I:C) activates TLR3 and MDA5 to induce Th1 cell and CD8+ T-cell immune responses through the production of IFN and cytokines 172 . Activation of TLR7/8 and the RIG-I pathway promotes macrophage differentiation toward the M1 proinflammatory phenotype and exhibits antitumor activity 173 , 174 (Fig. 4a ). In summary, NA-sensing has emerged as a promising target for cancer immunotherapy 175 .
Negative regulation of nucleic acid sensing in therapy
The disadvantages of intrinsic NA-sensing activation are mainly observed in autoimmune and inflammatory diseases 176 . NA-sensing is especially important during the metastatic stage of cancer and is activated under specific conditions. Increased levels of inflammatory factors caused by NA-sensing have been associated with poor prognosis 8 (Fig. 4b ). Recent studies have shown that RIG-I attenuates the tumor-killing effect of CD8+ T cells by inhibiting STAT5 action 177 .
Although the innate immune response induced via nucleic acid immunity can contribute to disease attenuation, it also plays a negative regulatory role. NA-sensing induces apoptosis in host cells via multiple pathways 178 . IRF3-mediated apoptosis impairs T-cell proliferation and metabolism 179 . Mechanistically, activated IRF3 binds to the proapoptotic protein Bax, and the subsequent translocation of the IRF3-Bax complex to mitochondria promotes the release of cytochrome c into the cytoplasm, thereby inducing apoptosis 8 , 180 . In contrast, NA-sensing mediates the degradation of transfected RNA and inhibits translation initiation through the actions of interferons 181 . OAS recognizes dsRNA and activates RNase L to mediate RNA degradation. The degraded RNA can also activate other NA-sensing PRRs 182 . dsRNA-dependent activation of PKR subsequently phosphorylates translation initiation factor eIF2α, resulting in translation repression 183 . IFIT1 can also suppress translation by sequestering eukaryotic initiation factors or directly binding to the 5′ end of foreign RNA 184 (Fig. 4c ).
mRNA vaccines elicit different immune responses by encoding antigenic proteins. On the one hand, mRNA-encoded proteins acting as endogenous antigens are degraded by proteasomes into antigenic peptides and activate CD8+ T cells via MHC class I molecules. On the other hand, mRNA vaccine-encoded proteins secreted into extracellular compartments are internalized by antigen-presenting cells, which generate antigenic peptides by proteolysis in endosomes and are presented to CD4+ T cells via MHC class II molecules, which can induce cytokine secretion and stimulate B cells to activate humoral immune responses 185 (Fig. 4c ). The induction of these immune responses depends on the transfection efficiency of the mRNA vaccine and is inhibited mainly by negative regulatory effects mediated by NA-sensing 149 . NA-sensing also causes gene editing difficulty in some cells. Inhibition or evasion of NA-sensing can save nucleic acids from translational repression, thereby improving gene transfection efficiency and increasing the expression of functional protein products 186 , 187 .
Strategies to evade nucleic acid sensing
Small-molecule inhibitors and viral proteins.
Small-molecule inhibitors (see review 188 ) of DNA sensing pathways have potential therapeutic value in diseases with long-term activation of proinflammatory pathways, such as autoimmune and inflammatory diseases 161 , 189 , 190 . In addition, A151 ODN inhibits the activity of multiple DNA receptors 191 , and 2′-O-methyl (2′OMe) gapmer-modified antisense oligonucleotides show sequence-dependent inhibition of NA-sensing mediated via RNase-H1 recruitment 192 .
Understanding how viruses evade immune recognition is important for antiviral research and immunotherapy 193 . Multiple virus-encoded proteins inhibit NA-sensing-associated pathways (see review 194 ). Vaccinia virus (VACV), the most studied Poxviridae 195 , degrades cGAMP via B2R gene-encoded POXIN 196 . Some viruses are thought to improve the efficiency of nucleic acid vaccines by blocking the RNA-sensing pathway and enhancing gene expression 197 . Among these proteins, influenza A virus nonstructural protein 1 (NS1) stimulates mRNA translation by inhibiting interferon production 198 . Vaccinia protein B18R inhibits type I IFN to enhance mRNA stability and translation efficiency 199 .
Sequence optimization and chemical modifications
Nucleic acid modification can prevent the innate immune response-mediated translational repression of exogenous genes by reducing immunogenicity 186 . The 5′-cap1 structure (a 2′-O-methyl group linked to the first nucleotide: m7GpppNmpN) can escape RIG-I recognition, thereby increasing translation efficiency 200 , 201 . The addition of poly(A) tails minimizes mRNA immunogenicity by reducing the U content of the sequence 186 . Circular RNAs (circRNAs) reportedly exhibit low immunogenicity and high stability and can initiate stable translation via internal ribosome entry site elements 202 , 203 . The incorporation of N6-methyladenosine (m6A)-modified circRNAs completely abrogated RIG-I-mediated activation of the immune response 204 . In addition, many chemical modifications of RNA bases have been leveraged to reduce the immunogenicity of mRNA; these modifications include pseudouridine (Ψ), N1-methyl-pseudouridine (m1Ψ), 2-thiouridine (s2U), 5-methoxyuridine (m5U), and 5-methylcytidine (m5C) 205 , 206 , 207 . DNA transfection was performed to construct CAR-modified immune cells, and the low efficiency of pDNA transfection in immune cells was appropriately resolved by removing CpG sequences and reducing plasmid size 208 .
Limitations and prospects of nucleic acid sensing in therapy
Despite multiple modifications aimed at limiting undesired immune stimulation caused by nucleic acid vaccines, further optimization is necessary to achieve the desired transfection efficiency and economic viability. For instance, although DNA vaccines can trigger an immune response in animal experiments, they exhibit low immunogenicity in human clinical trials 147 , thereby slowing the development of DNA vaccines. DNA vaccines have also been used in the fight against COVID-19; for example, the COVIDITY DNA vaccine was developed with two plasmids encoding the S protein receptor-binding domain and the nucleocapsid (N) protein, thus providing a mechanism to enhance extracellular antigen cross-presentation. Nevertheless, although physical delivery methods such as electroporation or needle-free injection systems may address delivery efficiency issues, the risk of DNA insertion remains a concern 209 . In contrast, agonists and antagonists of DNA sensors show more promise for clinical applications 6 . In contrast to DNA vaccines, RNA vaccines carry no risk of genomic insertion and are easy to deliver. Although balancing antigen expression and immunogenicity of RNA can increase the antigen availability, the thermal instability of these vaccines remains a challenge that has not been adequately addressed.
As mentioned earlier, NA-sensing activation exhibits both benefits and drawbacks in disease treatment, and its necessity must be carefully evaluated in the context of different diseases and stages of pathogenesis. The study and comparison of DNA-sensing and RNA-sensing interactions can help in identifying new optimization strategies 210 , 211 . The low immunogenicity of DNA vaccines may be due to some degree of cell-type specificity of DNA sensors, but it is unclear where nucleic acid vaccines that are injected into the skin accumulate. Targeted delivery of nucleic acid vaccines to lymph nodes or tumors may reduce NA-sensing while enhancing antigen-specific immune responses 212 . Furthermore, the effect of STING on tumor-associated macrophage differentiation helps alleviate tumor cell-mediated immunosuppression in the tumor microenvironment 213 . An alternative method for engineering T cells is in vivo RNA transfection 214 , although the role of NA-sensing of in vitro transcribed mRNA after CAR transfection remains unclear. A vast body of research links NA-sensing modulation to other therapeutic approaches 215 .
Conclusions
NA-sensing plays an important role in immunotherapy owing to its ability to elicit innate immunity. Therefore, a comprehensive understanding of the regulation and mechanisms underlying NA-sensing may contribute to the development of antitumor therapies. Several emerging regulatory mechanisms complement the profiling of NA-sensing systems. Although human nucleic acid receptors are diverse, their recognition ligands overlap, and there are similarities in their regulatory mechanisms and downstream signals, such as common adaptor proteins and cofactors. To effectively prevent pathogenic infections, humans have evolved redundant NA-sensing systems to complement the cellular recognition of immunogenic nucleic acids. Therefore, crosstalk among nucleic acid receptors is essential 216 . In this review, we describe the regulatory mechanisms of nucleic acid receptors.
NA-sensing is a double-edged sword in the field of therapeutics. In cancer therapy, NA-sensing tends to have a facilitative effect on antitumor immunity and is thus considered a potential treatment target. However, in the field of gene therapy, it is important to prevent the excessive activation of NA-sensing pathways to maintain proper immunogenicity and efficient gene transfection. The application of in vitro-transcribed mRNA has emerged as a promising therapeutic strategy. Multiple modification approaches have been proposed for increasing therapeutic efficiency by increasing transfection efficiency. In conclusion, nucleic acid sensors are potential targets for gene and cell therapies, which must be generated to balance therapeutic transgene-mediated innate and adaptive immune responses 145 , 217 , 218 .
Marshall, J. S., Warrington, R., Watson, W. & Kim, H. L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 14 , 49 (2018).
Article PubMed PubMed Central Google Scholar
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16 , 343–353 (2015).
Article CAS PubMed PubMed Central Google Scholar
Wang, Y. et al. cGAS-STING pathway in cancer biotherapy. Mol. Cancer 19 , 136 (2020).
Amouzegar, A., Chelvanambi, M., Filderman, J. N., Storkus, W. J. & Luke, J. J. STING agonists as cancer therapeutics. Cancers (Basel) 13 , https://doi.org/10.3390/cancers13112695 (2021).
Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA 114 , E4612–E4620 (2017).
McWhirter, S. M. & Jefferies, C. A. Nucleic acid sensors as therapeutic targets for human disease. Immunity 53 , 78–97 (2020).
Article CAS PubMed Google Scholar
Schlee, M. & Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 16 , 566–580 (2016).
Okude, H., Ori, D. & Kawai, T. Signaling through nucleic acid sensors and their roles in inflammatory diseases. Front. Immunol. 11 , 625833 (2020).
Kayraklioglu, N., Horuluoglu, B. & Klinman, D. M. CpG oligonucleotides as vaccine adjuvants. Methods Mol. Biol. 2197 , 51–85 (2021).
Zhang, C., Maruggi, G., Shan, H. & Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 10 , 594 (2019).
Berke, I. C., Li, Y. & Modis, Y. Structural basis of innate immune recognition of viral RNA. Cell Microbiol. 15 , 386–394 (2013).
Jelinek, I. et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 186 , 2422–2429 (2011).
Sakaniwa, K. et al. TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction. Nat. Commun. 14 , 164 (2023).
Miyake, K. et al. Nucleic acid sensing by toll-like receptors in the endosomal compartment. Front. Immunol. 13 , 941931 (2022).
Liu, G. & Gack, M. U. Distinct and orchestrated functions of RNA sensors in innate immunity. Immunity 53 , 26–42 (2020).
Kawasaki, T. & Kawai, T. Discrimination between self and non-self-nucleic acids by the innate immune system. Int Rev. Cell Mol. Biol. 344 , 1–30 (2019).
Rigby, R. E. et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 33 , 542–558 (2014).
Majer, O., Liu, B. & Barton, G. M. Nucleic acid-sensing TLRs: trafficking and regulation. Curr. Opin. Immunol. 44 , 26–33 (2017).
Lind, N. A., Rael, V. E., Pestal, K., Liu, B. & Barton, G. M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 22 , 224–235 (2022).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452 , 234–238 (2008).
Lee, B. L. & Barton, G. M. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 24 , 360–369 (2014).
Lee, B. L. et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife 2 , e00291 (2013).
Park, B. et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9 , 1407–1414 (2008).
Blasius, A. L. & Beutler, B. Intracellular toll-like receptors. Immunity 32 , 305–315 (2010).
Rimann, I. et al. The solute carrier SLC15A4 is required for optimal trafficking of nucleic acid-sensing TLRs and ligands to endolysosomes. Proc. Natl Acad. Sci. USA 119 , e2200544119 (2022).
Heinz, L. X. et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature 581 , 316–322 (2020).
Greulich, W. et al. TLR8 is a sensor of RNase T2 degradation products. Cell 179 , 1264–1275.e1213 (2019).
Liu, K. et al. Skewed endosomal RNA responses from TLR7 to TLR3 in RNase T2-deficient macrophages. Int. Immunol. 33 , 479–490 (2021).
Henneke, M. et al. RNASET2-deficient cystic leukoencephalopathy resembles congenital cytomegalovirus brain infection. Nat. Genet. 41 , 773–775 (2009).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166 , 88–101 (2016).
Wilber, A., Lu, M. & Schneider, M. C. Deoxyribonuclease I-like III is an inducible macrophage barrier to liposomal transfection. Mol. Ther. 6 , 35–42 (2002).
Al-Mayouf, S. M. et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat. Genet. 43 , 1186–1188 (2011).
Rodero, M. P. et al. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nat. Commun. 8 , 2176 (2017).
Gavin, A. L. et al. PLD3 and PLD4 are single-stranded acid exonucleases that regulate endosomal nucleic-acid sensing. Nat. Immunol. 19 , 942–953 (2018).
Yoshida, H., Okabe, Y., Kawane, K., Fukuyama, H. & Nagata, S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6 , 49–56 (2005).
Santa, P. et al. The role of nucleases and nucleic acid editing enzymes in the regulation of self-nucleic acid sensing. Front. Immunol. 12 , 629922 (2021).
Bertheloot, D. et al. RAGE enhances TLR responses through binding and internalization of RNA. J. Immunol. 197 , 4118–4126 (2016).
Ganguly, D. et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206 , 1983–1994 (2009).
Ivanov, S. et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110 , 1970–1981 (2007).
Nguyen, T. A. et al. SIDT1 localizes to endolysosomes and mediates double-stranded RNA transport into the cytoplasm. J. Immunol. 202 , 3483–3492 (2019).
Nguyen, T. A. et al. SIDT2 transports extracellular dsRNA into the cytoplasm for innate immune recognition. Immunity 47 , 498–509.e496 (2017).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32 , 461–488 (2014).
Kato, H. et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23 , 19–28 (2005).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5 , 730–737 (2004).
Kang, D. C. et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23 , 1789–1800 (2004).
Kowalinski, E. et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147 , 423–435 (2011).
Vabret, N. & Blander, J. M. Sensing microbial RNA in the cytosol. Front. Immunol. 4 , 468 (2013).
Sanchez David, R. Y. et al. Comparative analysis of viral RNA signatures on different RIG-I-like receptors. Elife 5 , e11275 (2016).
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205 , 1601–1610 (2008).
Wu, B. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152 , 276–289 (2013).
Liu, H. M. et al. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 11 , 528–537 (2012).
Lin, J. P., Fan, Y. K. & Liu, H. M. The 14-3-3eta chaperone protein promotes antiviral innate immunity via facilitating MDA5 oligomerization and intracellular redistribution. PLoS Pathog. 15 , e1007582 (2019).
Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 38 , 855–869 (2013).
Rothenfusser, S. et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175 , 5260–5268 (2005).
Saito, T. et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl Acad. Sci. USA 104 , 582–587 (2007).
Quicke, K. M., Kim, K. Y., Horvath, C. M. & Suthar, M. S. RNA helicase LGP2 negatively regulates RIG-I signaling by preventing TRIM25-mediated caspase activation and recruitment domain ubiquitination. J. Interferon Cytokine Res. 39 , 669–683 (2019).
Bruns, A. M., Leser, G. P., Lamb, R. A. & Horvath, C. M. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol. Cell 55 , 771–781 (2014).
Venkataraman, T. et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178 , 6444–6455 (2007).
Satoh, T. et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl Acad. Sci. USA 107 , 1512–1517 (2010).
Gack, M. U. et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446 , 916–920 (2007).
Oshiumi, H. et al. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe 8 , 496–509 (2010).
Yan, J., Li, Q., Mao, A. P., Hu, M. M. & Shu, H. B. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J. Mol. Cell Biol. 6 , 154–163 (2014).
Kuniyoshi, K. et al. Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity. Proc. Natl Acad. Sci. USA 111 , 5646–5651 (2014).
Shen, Y. et al. Riok3 inhibits the antiviral immune response by facilitating TRIM40-mediated RIG-I and MDA5 degradation. Cell Rep. 35 , 109272 (2021).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20 , 537–551 (2020).
Hu, M. M., Liao, C. Y., Yang, Q., Xie, X. Q. & Shu, H. B. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 214 , 973–989 (2017).
Gack, M. U., Nistal-Villan, E., Inn, K. S., Garcia-Sastre, A. & Jung, J. U. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 84 , 3220–3229 (2010).
Seo, J. et al. O-linked N-acetylglucosamine modification of mitochondrial antiviral signaling protein regulates antiviral signaling by modulating its activity. Front. Immunol. 11 , 589259 (2020).
Lui, P. Y. et al. PACT facilitates RNA-induced activation of MDA5 by promoting MDA5 oligomerization. J. Immunol. 199 , 1846–1855 (2017).
Kok, K. H. et al. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe 9 , 299–309 (2011).
Lian, H. et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity 49 , 438–448.e435 (2018).
Oshiumi, H. et al. DDX60 is involved in RIG-I-dependent and independent antiviral responses, and its function is attenuated by virus-induced EGFR activation. Cell Rep. 11 , 1193–1207 (2015).
Zhou, Z. et al. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response. Proc. Natl Acad. Sci. USA 111 , E245–E254 (2014).
Acharya, D. et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell 185 , 3588–3602.e3521 (2022).
Xu, L., Xiao, N., Liu, F., Ren, H. & Gu, J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc. Natl Acad. Sci. USA 106 , 1530–1535 (2009).
Paget, M. et al. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol. Cell 83 , 1180–1196.e1188 (2023).
Hao, Y. et al. ZBP1: a powerful innate immune sensor and double-edged sword in host immunity. Int. J. Mol. Sci. 23 , https://doi.org/10.3390/ijms231810224 (2022).
Chan, C. P. & Jin, D. Y. Cytoplasmic RNA sensors and their interplay with RNA-binding partners in innate antiviral response: theme and variations. RNA 28 , 449–477 (2022).
Bartok, E. & Hartmann, G. Immune sensing mechanisms that discriminate self from altered self and foreign nucleic acids. Immunity 53 , 54–77 (2020).
Samuel, C. E. Adenosine deaminase acting on RNA (ADAR1), a suppressor of double-stranded RNA-triggered innate immune responses. J. Biol. Chem. 294 , 1710–1720 (2019).
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7 , 40–48 (2006).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448 , 501–505 (2007).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138 , 576–591 (2009).
Hornung, V., Hartmann, R., Ablasser, A. & Hopfner, K. P. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 14 , 521–528 (2014).
Gentili, M. et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 26 , 2377–2393.e2313 (2019).
Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361 , 704–709 (2018).
Zhou, W., Mohr, L., Maciejowski, J. & Kranzusch, P. J. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol. Cell 81 , 739–755.e737 (2021).
Luecke, S. et al. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 18 , 1707–1715 (2017).
Xie, W. et al. Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc. Natl Acad. Sci. USA 116 , 11946–11955 (2019).
Siddiqui, M. A. & Yamashita, M. Toll-like receptor (TLR) signaling enables cyclic GMP-AMP synthase (cGAS) sensing of HIV-1 infection in macrophages. mBio 12 , e0281721 (2021).
Article PubMed Google Scholar
Yoh, S. M. et al. Recognition of HIV-1 capsid by PQBP1 licenses an innate immune sensing of nascent HIV-1 DNA. Mol. Cell 82 , 2871–2884.e2876 (2022).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11 , 997–1004 (2010).
Morrone, S. R. et al. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc. Natl Acad. Sci. USA 111 , E62–E71 (2014).
Jiang, Z. et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat. Microbiol 6 , 932–945 (2021).
Sun, H. et al. A nuclear export signal is required for cGAS to sense cytosolic DNA. Cell Rep. 34 , 108586 (2021).
Almine, J. F. et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 8 , 14392 (2017).
Warga, E., Anderson, J., Tucker, M., Harris, E. & Elmer, J. Transcriptomic analysis of the innate immune response to in vitro transfection of plasmid DNA. Mol. Ther. Nucleic Acids 31 , 43–56 (2023).
Yum, S., Li, M., Fang, Y. & Chen, Z. J. TBK1 recruitment to STING activates both IRF3 and NF-kappaB that mediate immune defense against tumors and viral infections. Proc. Natl Acad. Sci. USA 118 , https://doi.org/10.1073/pnas.2100225118 (2021).
Lee, M. N. et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat. Immunol. 14 , 179–185 (2013).
Hertzog, J. & Rehwinkel, J. Regulation and inhibition of the DNA sensor cGAS. EMBO Rep. 21 , e51345 (2020).
Gao, D. et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112 , E5699–E5705 (2015).
Pokatayev, V. et al. RNase H2 catalytic core Aicardi-Goutieres syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J. Exp. Med. 213 , 329–336 (2016).
Schumann, T. et al. Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent manner. J. Exp. Med. 220 , https://doi.org/10.1084/jem.20220829 (2023).
Deng, Y., Wang, Y., Li, L., Miao, E. A. & Liu, P. Post-translational modifications of proteins in cytosolic nucleic acid sensing signaling pathways. Front. Immunol. 13 , 898724 (2022).
Chen, M. et al. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol. Cell 64 , 105–119 (2016).
Yang, X. et al. MARCH8 attenuates cGAS-mediated innate immune responses through ubiquitylation. Sci. Signal 15 , eabk3067 (2022).
Cai, X. et al. Opposing effects of deubiquitinase OTUD3 in innate immunity against RNA and DNA viruses. Cell Rep. 39 , 110920 (2022).
Song, Z. M. et al. KAT5 acetylates cGAS to promote innate immune response to DNA virus. Proc. Natl Acad. Sci. USA 117 , 21568–21575 (2020).
Dai, J. et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell 176 , 1447–1460.e1414 (2019).
Hu, M. M. et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity 45 , 555–569 (2016).
Cui, Y. et al. SENP7 potentiates cGAS activation by relieving SUMO-mediated inhibition of cytosolic DNA sensing. PLoS Pathog. 13 , e1006156 (2017).
Seo, G. J. et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 13 , 440–449 (2015).
Sun, X. et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat. Commun. 11 , 6182 (2020).
Wang, X. et al. Aurora A kinase inhibition compromises its antitumor efficacy by elevating PD-L1 expression. J. Clin. Invest. 133 , https://doi.org/10.1172/JCI161929 (2023).
Wang, Y. et al. The role of O-GlcNAcylation in innate immunity and inflammation. J. Mol. Cell Biol. 14 , https://doi.org/10.1093/jmcb/mjac065 (2023).
Hu, J. et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 11 , 805–823 (2021).
Li, T., Diner, B. A., Chen, J. & Cristea, I. M. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl Acad. Sci. USA 109 , 10558–10563 (2012).
Baker, P. J. et al. Posttranslational modification as a critical determinant of cytoplasmic innate immune recognition. Physiol. Rev. 97 , 1165–1209 (2017).
Li, D. et al. IFI16 isoforms with cytoplasmic and nuclear locations play differential roles in recognizing invaded DNA viruses. J. Immunol. 207 , 2699–2709 (2021).
Zhao, M. et al. The stress granule protein G3BP1 promotes pre-condensation of cGAS to allow rapid responses to DNA. EMBO Rep. 23 , e53166 (2022).
Zhang, J. et al. ZYG11B potentiates the antiviral innate immune response by enhancing cGAS-DNA binding and condensation. Cell Rep. 42 , 112278 (2023).
Tao, X. et al. Ku proteins promote DNA binding and condensation of cyclic GMP-AMP synthase. Cell Rep. 40 , 111310 (2022).
Lian, H. et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 9 , 3349 (2018).
Singh, R. S. et al. DDX41 is required for cGAS-STING activation against DNA virus infection. Cell Rep. 39 , 110856 (2022).
Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA 106 , 20842–20846 (2009).
Lv, T. et al. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J. Immunother. Cancer 10 , https://doi.org/10.1136/jitc-2022-004763 (2022).
Ghosh, A. et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity 50 , 51–63.e55 (2019).
Liao, C. Y., Lei, C. Q. & Shu, H. B. PCBP1 modulates the innate immune response by facilitating the binding of cGAS to DNA. Cell Mol. Immunol. 18 , 2334–2343 (2021).
Gu, H. et al. PCBP2 maintains antiviral signaling homeostasis by regulating cGAS enzymatic activity via antagonizing its condensation. Nat. Commun. 13 , 1564 (2022).
Orzalli, M. H. et al. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc. Natl Acad. Sci. USA 112 , E1773–E1781 (2015).
Jonsson, K. L. et al. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat. Commun. 8 , 14391 (2017).
Li, D. et al. STING-mediated IFI16 degradation negatively controls type I interferon production. Cell Rep. 29 , 1249–1260.e1244 (2019).
Luteijn, R. D. et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573 , 434–438 (2019).
Cordova, A. F., Ritchie, C., Bohnert, V. & Li, L. Human SLC46A2 IS the Dominant cGAMP importer in extracellular cGAMP-sensing macrophages and monocytes. ACS Cent. Sci. 7 , 1073–1088 (2021).
Zhou, C. et al. Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity 52 , 767–781.e766 (2020).
Wei, X. et al. LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep. 39 , 110880 (2022).
Zhou, Y. et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity 52 , 357–373.e359 (2020).
Pepin, G. et al. Connexin-dependent transfer of cGAMP to phagocytes modulates antiviral responses. mBio 11 , https://doi.org/10.1128/mBio.03187-19 (2020).
Maltbaek, J. H., Cambier, S., Snyder, J. M. & Stetson, D. B. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity 55 , 1799–1812.e1794 (2022).
Carozza, J. A. et al. ENPP1’s regulation of extracellular cGAMP is a ubiquitous mechanism of attenuating STING signaling. Proc. Natl Acad. Sci. USA 119 , e2119189119 (2022).
Zahid, A., Ismail, H., Li, B. & Jin, T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front Immunol. 11 , 613039 (2020).
Fuertes, M. B., Woo, S. R., Burnett, B., Fu, Y. X. & Gajewski, T. F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 34 , 67–73 (2013).
Suter, M. A. et al. cGAS-STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells. Sci. Rep. 11 , 7243 (2021).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247 , 1465–1468 (1990).
Miao, L., Zhang, Y. & Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 20 , 41 (2021).
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451 , 725–729 (2008).
Coban, C. et al. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther. 11 , 479–484 (2011).
Kobiyama, K. & Ishii, K. J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 23 , 474–476 (2022).
Mu, X. & Hur, S. Immunogenicity of In Vitro-Transcribed RNA. Acc. Chem. Res 54 , 4012–4023 (2021).
Sprent, J. & King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci Immunol 6 , https://doi.org/10.1126/sciimmunol.abj9256 (2021).
Miura, N., Shaheen, S. M., Akita, H., Nakamura, T. & Harashima, H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 43 , 1317–1331 (2015).
Li, A. et al. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 12 , 35 (2019).
Jeremiah, N. et al. RELA tunes innate-like interferon I/III responses in human T cells. J. Exp. Med. 220 , https://doi.org/10.1084/jem.20220666 (2023).
Marcus, A. et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity 49 , 754–763 .e754 (2018).
Schadt, L. et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 29 , 1236–1248.e1237 (2019).
Feng, X. et al. ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 39 , e104036 (2020).
Du, S. S. et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J. Radiat. Oncol. Biol. Phys. 112 , 1243–1255 (2022).
Jesenko, T. et al. Radiation induced upregulation of DNA sensing pathways is cell-type dependent and can mediate the off-target effects. Cancers (Basel) 12 , https://doi.org/10.3390/cancers12113365 (2020).
Tian, Z., Zeng, Y., Peng, Y., Liu, J. & Wu, F. Cancer immunotherapy strategies that target the cGAS-STING pathway. Front. Immunol. 13 , 996663 (2022).
Jiang, Y. et al. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J. Hematol. Oncol. 16 , 8 (2023).
Skopelja-Gardner, S., An, J. & Elkon, K. B. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat. Rev. Nephrol. 18 , 558–572 (2022).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7 , 283ra252 (2015).
Article Google Scholar
Heidegger, S. et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade. EBioMedicine 41 , 146–155 (2019).
Liu, J. et al. PRMT1 mediated methylation of cGAS suppresses anti-tumor immunity. Nat. Commun. 14 , 2806 (2023).
Ma, D. et al. Arginine methyltransferase PRMT5 negatively regulates cGAS-mediated antiviral immune response. Sci. Adv. 7 , https://doi.org/10.1126/sciadv.abc1834 (2021).
Boehmer, D. F. R. et al. OAS1/RNase L executes RIG-I ligand-dependent tumor cell apoptosis. Sci. Immunol. 6 , https://doi.org/10.1126/sciimmunol.abe2550 (2021).
Tudor, D. et al. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 23 , 1258–1264 (2005).
Moseman, E. A. et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 173 , 4433–4442 (2004).
Baghban, R., Ghasemian, A. & Mahmoodi, S. Nucleic acid-based vaccine platforms against the coronavirus disease 19 COVID-19. Arch. Microbiol. 205 , 150 (2023).
Stevenson, E. M. et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8(+) T-cells. Nat. Commun. 13 , 4888 (2022).
Hemann, E. A. et al. A Small Molecule RIG-I Agonist Serves as an Adjuvant to Induce Broad Multifaceted Influenza Virus Vaccine Immunity. J. Immunol. 210 , 1247–1256 (2023).
Apostolico, J. S. et al. Poly(I:C) potentiates T cell immunity to a dendritic cell targeted HIV-multiepitope vaccine. Front. Immunol. 10 , 843 (2019).
Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2 , 578–588 (2018).
Zhou, B., Li, C., Yang, Y. & Wang, Z. RIG-I promotes cell death in hepatocellular carcinoma by inducing M1 polarization of perineal macrophages through the RIG-I/MAVS/NF-kappaB pathway. Onco Targets Ther. 13 , 8783–8794 (2020).
Yang, R., Yu, S., Xu, T., Zhang, J. & Wu, S. Emerging role of RNA sensors in tumor microenvironment and immunotherapy. J. Hematol. Oncol. 15 , 43 (2022).
Crowl, J. T., Gray, E. E., Pestal, K., Volkman, H. E. & Stetson, D. B. Intracellular nucleic acid detection in autoimmunity. Annu. Rev. Immunol. 35 , 313–336 (2017).
Jiang, X. et al. Intrinsic RIG-I restrains STAT5 activation to modulate antitumor activity of CD8+ T cells. J. Clin. Invest. 133 , https://doi.org/10.1172/JCI160790 (2023).
Zheng, W. et al. How the innate immune DNA sensing cGAS-STING pathway is involved in apoptosis. Int. J. Mol. Sci. 24 , https://doi.org/10.3390/ijms24033029 (2023).
Kuhl, N. et al. STING agonism turns human T cells into interferon-producing cells but impedes their functionality. EMBO Rep. 24 , e55536 (2023).
Murthy, A. M. V., Robinson, N. & Kumar, S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differ. 27 , 2989–3003 (2020).
Gu, C. et al. Transfected DNA is targeted by STING-mediated restriction. Biochem. Biophys. Res. Commun. 549 , 207–213 (2021).
Burke, J. M. et al. RNase L activation in the cytoplasm induces aberrant processing of mRNAs in the nucleus. PLoS Pathog. 18 , e1010930 (2022).
Zhang, F. et al. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 276 , 24946–24958 (2001).
Fleith, R. C. et al. IFIT3 and IFIT2/3 promote IFIT1-mediated translation inhibition by enhancing binding to non-self RNA. Nucleic Acids Res. 46 , 5269–5285 (2018).
Fang, E. et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target Ther. 7 , 94 (2022).
Kim, S. C. et al. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell Toxicol. 18 , 1–8 (2022).
Yang, B., Jeang, J., Yang, A., Wu, T. C. & Hung, C. F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 10 , 3153–3164 (2014).
Lama, L. et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat. Commun. 10 , 2261 (2019).
Zhang, S., Zheng, R., Pan, Y. & Sun, H. Potential Therapeutic Value of the STING Inhibitors. Molecules 28 , https://doi.org/10.3390/molecules28073127 (2023).
Wiser, C., Kim, B., Vincent, J. & Ascano, M. Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells. Sci. Rep. 10 , 7604 (2020).
Steinhagen, F. et al. Suppressive oligodeoxynucleotides containing TTAGGG motifs inhibit cGAS activation in human monocytes. Eur. J. Immunol. 48 , 605–611 (2018).
Valentin, R. et al. Sequence-dependent inhibition of cGAS and TLR9 DNA sensing by 2’-O-methyl gapmer oligonucleotides. Nucleic Acids Res. 49 , 6082–6099 (2021).
Yu, H., Bruneau, R. C., Brennan, G. & Rothenburg, S. Battle royale: innate recognition of poxviruses and viral immune evasion. Biomedicines 9 , https://doi.org/10.3390/biomedicines9070765 (2021).
Chan, Y. K. & Gack, M. U. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol 14 , 360–373 (2016).
El-Jesr, M., Teir, M. & Maluquer de Motes, C. Vaccinia virus activation and antagonism of cytosolic DNA sensing. Front. Immunol. 11 , 568412 (2020).
Eaglesham, J. B., Pan, Y., Kupper, T. S. & Kranzusch, P. J. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature 566 , 259–263 (2019).
Jaafar, Z. A. & Kieft, J. S. Viral RNA structure-based strategies to manipulate translation. Nat. Rev. Microbiol 17 , 110–123 (2019).
Koliopoulos, M. G. et al. Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition. Nat. Commun. 9 , 1820 (2018).
Minnaert, A. K. et al. Vaccinia virus protein B18R: influence on mRNA immunogenicity and translation upon non-viral delivery in different ocular cell types. Pharmaceutics 13 , https://doi.org/10.3390/pharmaceutics13010074 (2021).
Wuebben, C., Bartok, E. & Hartmann, G. Innate sensing of mRNA vaccines. Curr. Opin. Immunol. 79 , 102249 (2022).
Devarkar, S. C. et al. Structural basis for m7G recognition and 2’-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl Acad. Sci. USA 113 , 596–601 (2016).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9 , 2629 (2018).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell 74 , 508–520.e504 (2019).
Chen, Y. G. et al. N6-methyladenosine modification controls circular RNA immunity. Mol. Cell 76 , 96–109.e109 (2019).
Moradian, H., Roch, T., Anthofer, L., Lendlein, A. & Gossen, M. Chemical modification of uridine modulates mRNA-mediated proinflammatory and antiviral response in primary human macrophages. Mol. Ther. Nucleic Acids 27 , 854–869 (2022).
Wang, Y. et al. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer 20 , 33 (2021).
Mei, Y. & Wang, X. RNA modification in mRNA cancer vaccines. Clin. Exp. Med. 23 , 1917–1931 (2023).
Cha, E. B., Shin, K. K., Seo, J. & Oh, D. B. Antibody-secreting macrophages generated using CpG-free plasmid eliminate tumor cells through antibody-dependent cellular phagocytosis. BMB Rep. 53 , 442–447 (2020).
Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 39 , 1479–1482 (2021).
Tse, S. W. et al. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 29 , 2227–2238 (2021).
Chen, Z. et al. An mRNA vaccine elicits STING-dependent antitumor immune responses. Acta Pharm. Sin. B 13 , 1274–1286 (2023).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl Acad. Sci. USA 119 , e2207841119 (2022).
Wang, Q. et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat. Commun. 13 , 3022 (2022).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11 , 6080 (2020).
Johnson, L. R. et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184 , 4981–4995.e4914 (2021).
Anwar, S., Ul Islam, K., Azmi, M. I. & Iqbal, J. cGAS-STING-mediated sensing pathways in DNA and RNA virus infections: crosstalk with other sensing pathways. Arch. Virol. 166 , 3255–3268 (2021).
Linares-Fernandez, S., Lacroix, C., Exposito, J. Y. & Verrier, B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 26 , 311–323 (2020).
Colombani, T., Haudebourg, T. & Pitard, B. 704/DNA vaccines leverage cytoplasmic DNA stimulation to promote anti-HIV neutralizing antibody production in mice and strong immune response against alpha-fetoprotein in non-human primates. Mol. Ther. Nucleic Acids 32 , 743–757 (2023).
Download references
Acknowledgements
This work was supported by the National Research Foundation grant (2022M3E5F1016693) and the National Research Council of Science and Technology (NST) grant (CAP-18-02-KRIBB) by the Korean government. We sincerely appreciate laboratory members for helpful discussions in preparing this manuscript.
Author information
Authors and affiliations.
Immunotherapy Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, 34141, Republic of Korea
Ling-Zu Kong, Seok-Min Kim, Chunli Wang, Soo Yun Lee, Se-Chan Oh, Sunyoung Lee, Seona Jo & Tae-Don Kim
Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon, 34134, Republic of Korea
Ling-Zu Kong
Department of Life Sciences, Korea University, Seoul, 02841, Korea
Sunyoung Lee
Department of Functional Genomics, KRIBB School of Bioscience, Korea University of Science and Technology (UST), Daejeon, 34113, Korea
Seona Jo & Tae-Don Kim
Biomedical Mathematics Group, Institute for Basic Science (IBS), Daejeon, Republic of Korea
Tae-Don Kim
Department of Biopharmaceutical Convergence, School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea
You can also search for this author in PubMed Google Scholar
Contributions
T.D.K. and L.Z.K. conceived the manuscript. L.Z.K. drafted the manuscript. All authors reviewed the manuscript. S.M.K, C.L.W. modified the manuscript. T.D.K. supervised manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Tae-Don Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kong, LZ., Kim, SM., Wang, C. et al. Understanding nucleic acid sensing and its therapeutic applications. Exp Mol Med 55 , 2320–2331 (2023). https://doi.org/10.1038/s12276-023-01118-6
Download citation
Received : 29 June 2023
Revised : 16 August 2023
Accepted : 20 August 2023
Published : 09 November 2023
Issue Date : November 2023
DOI : https://doi.org/10.1038/s12276-023-01118-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Porcine deltacoronavirus nonstructural protein 2 inhibits type i and iii ifn production by targeting sting for degradation.
Veterinary Research (2024)
Epigenetic silencing of ZIC4 unveils a potential tumor suppressor role in pediatric choroid plexus carcinoma
- Dina Hesham
- Amal Mosaab
- Shahenda El-Naggar
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
The 2022 Nucleic Acids Research database issue and the online molecular biology database collection
Daniel j rigden, xosé m fernández.
- Author information
- Article notes
- Copyright and License information
To whom correspondence should be addressed. Tel: +44 151 795 4467; Email: [email protected]
Collection date 2022 Jan 7.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
The 2022 Nucleic Acids Research Database Issue contains 185 papers, including 87 papers reporting on new databases and 85 updates from resources previously published in the Issue. Thirteen additional manuscripts provide updates on databases most recently published elsewhere. Seven new databases focus specifically on COVID-19 and SARS-CoV-2, including SCoV2-MD, the first of the Issue's Breakthrough Articles. Major nucleic acid databases reporting updates include MODOMICS, JASPAR and miRTarBase. The AlphaFold Protein Structure Database, described in the second Breakthrough Article, is the stand-out in the protein section, where the Human Proteoform Atlas and GproteinDb are other notable new arrivals. Updates from DisProt, FuzDB and ELM comprehensively cover disordered proteins. Under the metabolism and signalling section Reactome, ConsensusPathDB, HMDB and CAZy are major returning resources. In microbial and viral genomes taxonomy and systematics are well covered by LPSN, TYGS and GTDB. Genomics resources include Ensembl, Ensembl Genomes and UCSC Genome Browser. Major returning pharmacology resource names include the IUPHAR/BPS guide and the Therapeutic Target Database. New plant databases include PlantGSAD for gene lists and qPTMplants for post-translational modifications. The entire Database Issue is freely available online on the Nucleic Acids Research website ( https://academic.oup.com/nar ). Our latest update to the NAR online Molecular Biology Database Collection brings the total number of entries to 1645. Following last year's major cleanup, we have updated 317 entries, listing 89 new resources and trimming 80 discontinued URLs. The current release is available at http://www.oxfordjournals.org/nar/database/c/ .
NEW AND UPDATED DATABASES
The 29th annual Nucleic Acids Research Database Issue contains 185 papers covering topics from across biology and beyond. The ongoing COVID-19 pandemic continues to play a major role, inspiring the construction of seven new databases (Table 1 ). The reader will also find its impact obvious in papers describing other new and returning databases throughout the Issue. A further 80 papers (Table 2 ) report on other new databases while returning databases contribute a further 85 papers. Finally, there are 13 papers from resources most recently published elsewhere (Table 3 ).
Descriptions of new databases related to COVID-19 in the 2022 NAR Database issue
Descriptions of new databases in the 2022 NAR Database issue not specifically related to COVID-19
Updated descriptions of databases most recently published elsewhere
As usual, the Issue begins with updates from the major database providers at the European Bioinformatics Institute (EBI), the U.S. National Center for Biotechnology Information (NCBI), and the National Genomics Data Center (NGDC) in China ( 1–3 ). Thereafter, articles are placed in the usual categories: (i) nucleic acid sequence, structure and transcriptional regulation; (ii) protein sequence and structure; (iii) metabolic and signaling pathways, enzymes and networks; (iv) genomics of viruses, bacteria, protozoa and fungi; (v) genomics of human and model organisms plus comparative genomics; (vi) human genomic variation, diseases and drugs; (vii) plants and (viii) other topics, such as proteomics databases. As ever, many databases straddle multiple categories and readers are encouraged to check the full list of papers.
The COVID-19 papers include the SCoV2-MD publication ( 4 ) that is the first ‘Breakthrough’ Article in the Issue. NAR assigns Breakthrough status to papers that solve long-standing problems, or which are otherwise considered of exceptional importance. SCoV2-MD archives Molecular Dynamics simulations of all experimentally determined SARS-CoV-2 proteins. Impressively linked to phylogenetic data, it also enables users to consider the potential impact of variants on protein structure-function considering not only the usual static metrics, but also scores deriving from trajectory analysis. Elsewhere the Ensembl COVID-19 resource ( 5 ) places the SARS-CoV-2 genome in the familiar Ensembl framework, providing evolutionary insights and integrating information regarding non-coding RNA structures (from Rfam ( 6 )) and variants. Other COVID-19 databases cover transcriptomics of infected cells, both in SCovid ( 7 ) from a single cell perspective that allows a tissue-specific view of infection and in COVID19db ( 8 ) with an emphasis on network analysis and opportunities for drug discovery. The final three databases consider the immune response to infection and the potential impact of viral genomic variants on its effectiveness. The T-cell COVID-19 Atlas ( 9 ) predicts the affinity of interaction between virus-derived peptides and HLA alleles, potentially helping to predict the susceptibility of people with different HLA genotypes to disease. Finally, ESC ( 10 ) is a compilation of SARS-CoV-2 variants with documented effects on antibody binding while VarEPS ( 11 ) considers a number of metrics, including antibody binding, in order to predict the potential impact of all possible SARS-CoV-2 variants.
In the ‘ Nucleic acid databases ’ section, several resources illustrate the trend towards single cell-level data acquisition. Two databases cover alternative polyadenylation (APA): scAPAatlas ( 12 ) offers comprehensive analysis of human and mouse data, including correlation with gene expression and links to RNA-binding proteins or miRNAs on APA-regulated regions; scAPAdb ( 13 ) extends covered species to Arabidopsis and other plants. Elsewhere scEnhancer ( 14 ) offers a single cell perspective of enhancer regions in model organisms while scMethBank ( 15 ) covers DNA methylation in human and mouse and in healthy or cancerous cells, extending the whole organism data previously captured by the same group in MethBank ( 16 ).
Following last year's flurry of databases on proteins implicated in liquid–liquid phase separation, this year sees two new resources, RNAPhaSep and RPS ( 17 , 18 ), capturing information on RNA molecules implicated in this phenomenon. Each curates information on experimental data and links implicated RNA molecules to information on sequence, structure, interactions, disease associations and so on. These data are hosted at popular resources including RNAInter ( 19 ) and RNALocate ( 20 ), each reporting updates this year. Transcription factors (TFs) and their binding sites are well-covered this year. The heavily used JASPAR database ( 21 ) reports a particular focus on plant TF domains as well as the introduction of word clouds as a clever visualisation of functions linked to a given TF. Factorbook ( 22 ) returns after a number of years to focus on interpretation of SNPs lying within TF-binding motifs and to facilitate downstream AI analyses with convenient Numpy format downloads. The various relationships between TFs and cell markers are described in the new database TF-Marker ( 23 ), and the same group also describe TcoFBbase ( 24 ) covering transcription cofactors and associated regulatory networks. Elsewhere, notable returning databases include MODOMICS ( 25 ) which now links to PDB structures containing modified RNA and has improved associations between RNA modification and disease; miRTarBase ( 26 ) which updates content significantly and includes new features such as editing and disease-related variants; and miRNATissueAtlas ( 27 ) which switches from microarray-based analysis to deep sequencing and expands the number of donors and tissues to give a higher resolution picture of the tissue specificity of miRNA expression.
The section on ‘ Protein sequence and structure databases ’ begins with the Issue's second ‘Breakthrough Article’. After its dramatic emergence at the most recent CASP competition ( 28 ) the AlphaFold 2 (AF2) software for protein structure prediction was quickly published ( 29 ) released open source ( https://github.com/deepmind/alphafold ) and applied to the complete human proteome ( 30 ). Shortly after, the AlphaFold Protein Structure Database, described here ( 31 ), was released and covers 21 proteomes. The high-quality predicted structures in the database, projected to ultimately cover UniRef90 ( 32 ) protein sequence space, provide a treasure chest of information across all aspects of biology. The impact of the database, and the software more broadly, is reflected in the incorporation of its models into cornerstone resources such as UniProt ( 33 ) and InterPro ( 34 ) but also the rapid inclusion of AF2 outputs in a number of other databases in this Issue. AF2 models and other predicted structures are now included, for example, in PDBe-KB ( 35 ) which thus graphically illustrates the complementarity between experimental structures and computational models.
Other notable new databases include the Human Proteoform Atlas ( 36 ) which assigns stable identifiers to over 37 000 proteoforms, i.e. the different protein forms that can arise combinatorially from a single gene as a result of alternative splicing, coding sequence variants and post-translational modifications. Elsewhere, the GproteinDb ( 37 ) curates a wealth of information, especially information on the selectivity of their coupling to GPCRs, for a family of great importance to therapeutic design. Among databases reporting updates is PRIDE ( 38 ) where around 500 proteomics datasets are processed each month. After processing by improved data pipelines, the results are increasingly disseminated to other key databases such as UniProt ( 33 ), Ensembl ( 39 ) and Expression Atlas ( 40 ). Other returning databases focus on proteins or protein regions lacking a single, conventionally folded structure. DisProt ( 41 ), the database for intrinsically disordered protein, reports interestingly on the nuts and bolts of curation, harnessing both professional and community biocurators in a manner supported by a refactored ontology and incentivised by the APICURON database ( 42 ). The FuzDB Update ( 43 ) reports on fuzzy interactions, i.e. those exhibiting context-dependent conformational heterogeneity, an interaction style particularly common where one or both partners are classified as intrinsically disordered. FuzDB has a new interface and expanded links out to databases covering protein structure, function and involvement in phase separation. Short linear interaction motifs are particularly common in intrinsically disordered regions and the database for such motifs in eukaryotes, ELM, contributes an Update paper ( 44 ). Among highlighted examples of newly catalogued motifs, the authors use a KEGG ( 45 ) image of endocytosis pathways to emphasise the ubiquity of motif-mediated interactions in the process and illustrate the multiple points at which diverse viruses hijack pathway components. The paper also includes an interesting window onto the variety of databases and tools used by ELM curators to sift likely real motifs from false positive matches to regular expressions.
In the ‘ Metabolic and signalling pathways ’ section, the popular Reactome database of biological processes and networks has an Update paper ( 46 ) describing an interesting collaboration with the ‘Illuminating the Druggable Genome’ (IDG) consortium ( 47 ) that helps place many ‘dark’ proteins (those that are poorly understood and/or understudied) in the context of Reactome networks. The paper also reports curation of the processes behind SARS-CoV-2 infection, a procedure interestingly expedited by first working on SAR-CoV-1 from March 2020. Reactome is one of 31 resources contributing to the molecular interaction meta-resource ConsensusPathDB which also has an Update paper ( 48 ) reporting a quadrupling in size. Options for enrichment analysis in gene set queries of the network now include regulators such as miRNA and transcription factors. Other new databases include Kincore ( 49 ), a resource that classifies protein kinase conformations and ligand types, improving our understanding of the conformational landscape of this important family and facilitating drug design. Interestingly, AlphaFold Database predictions are included and classified alongside experimental structures. Among returning databases, HMDB, the Human Metabolome Database, reports ( 50 ) a near-doubling in size, intense recuration of hundreds of the most significant metabolites, more accurately predicted spectra and improved Pathway illustrations mapping metabolites onto anatomical and (sub)-cellular features. Elsewhere, an Update paper from CAZy ( 51 ), the database of carbohydrate-active enzymes, reports significant increases in numbers of enzyme families alongside interface improvements including Krona charts ( 52 ) for taxonomic distributions of families. Finally, sister EBI resources for macromolecular interactions IntAct ( 53 ) and Complex Portal ( 54 ) each contribute an Update. IntAct has more than doubled in size since its previous publication and captures diverse information on binary molecular interactions, including a SARS-CoV-2 interactome, in particularly clean and appealing visualisations. Complex Portal, as the name suggests, focuses on stable interactions between two or more macromolecules. It has, since last publication, focused on SARS-CoV-2 and on the 300 or so complexes believed to exist in Escherichia coli . Ongoing work is addressing human complexes which may number around 4000.
The ‘ Microbial genomics ’ section contains Update papers from three very significant taxonomy and systematics resources most recently published elsewhere. The resources LPSN (List of Prokaryotic names with Standing in Nomenclature) and TYGS (Type Strain Genome Server) publish together ( 55 ) and describe how their colocation in 2020 facilitates data exchange and mapping between them. The paper describes the ever-increasing pace of their growth and new options for genome-scale comparison of uploaded genomes to the sequences stored in TYGS. GTDB ( 56 ) is a regularly updating genome-based taxonomy for prokaryotes which reports on a trebling of species clusters since the last publication and on possibilities to move beyond INSDC genome sequences ( 57 ) to resources such as MGnify ( 58 ) in order to better capture the full scope of metagenome-assembled genomes now available on a large scale. Several new databases focus on microbiomes and metagenomes: mBodyMap ( 59 ) helps understand the prevalence and abundance of different bacteria at different sites on the human body in health and disease; gutMGene ( 60 ) curates information on gut microbiome metabolites and human target genes with which they interact; and AMDB ( 61 ) contains gut microbe information for almost 500 animal species. Three notable databases focus on host-pathogen interactions. The well-known PHI-BASE reports ( 62 ) new pathogens and hosts, and describes the range of other databases to which it contributes annotations. The second, VEuPathDB ( 63 ), is a new name to the Issue but contains genomic and a wide variety of other information on eukaryotic pathogens, their vectors and host, information previously stored in its parent databases VectorBase ( 64 ) and EuPathDB ( 65 ), each published here. The site allows construction of sophisticated search strategies and options for analysing host-pathogen interactions are a future priority. The third, the popular VFDB ( 66 ), returns with a novel hierarchical classification of its bacterial virulence factors (VFs) into 14 categories and >100 subcategories. Chromosome maps and genomic loci can be visualised with VFs colour-coded according to their categorisation. Finally, although not focused primarily on COVID-19, two databases include it among broader information that may well help predict the appearance and spread of future viral pandemics. VThunter ( 67 ) looks at expression of viral receptors at a single-cell level across 47 animal species enabling the users to ask which species a given virus might infect or, conversely, to which viruses a given animal might be susceptible. ZOVER ( 68 ) unites and upgrades two previous databases to curate information on zoonotic viruses carried by rodent, bat and insect vectors: information includes mapping of viral families to host species and geographical virus distributions.
In the next section (‘ Genomics of human and model organisms plus comparative genomics ’) a number of important databases contribute updates. Ensembl reports ( 69 ) on addressing the ever-increasing influx of data with new, more efficient workflows and a new Rapid Release platform which together allowed more than 200 genomes to be covered in around a year. A new interface is being implemented after researching user interaction patterns, and non-vertebrate genomes are also included for the first time as the database continues on the path to merger with Ensembl genomes. The paper on the latter ( 70 ) reports the largest content increase yet seen including almost 500 new fungal genomes. Other interesting developments include proteome-based removal of redundancy in hosted bacterial genomes, a move to better support pangenomes and inclusion of AlphaFold models for Arabidopsis. The USCS Genome Browser Update paper ( 71 ) describes a variety of new assemblies, tracks and display features, including support for different fonts in the genome browser display. There is also a clever SARS-CoV-2 feature allowing placement of a new genome in phylogenetic context, facilitating comparisons between sequences and with annotation tracks.
Elsewhere, a number of comparative genomics resources focusing on species of biological or agricultural importance feature. The Ruminant Genome Database ( 72 ) paper reports significant expansion of its multi-omics content throughout. Insects are the focus of three returning database: InsectBase ( 73 ) reports dramatic increases in content as well as new features focusing on ncRNA–mRNA interactions and likely horizontal gene transfer; Hymenoptera Genome Database ( 74 ) covers a tripling of covered species and a focus on better Gene Ontology ( 75 ) assignments allowing, for example, better on-site GO enrichment analysis; and FlyAtlas 2 ( 76 ) enhances its (sub-) tissue-specific gene expression data and introduces a new co-expression tool. As usual, aspects of human genomics feature strongly. The new PopHumanVar database ( 77 ) builds on previous work ( 78 , 79 ), calculating and assembling information on variants, in order to help identify those responsible for selective sweeps. 3DSNP ( 80 ), continues its work in contextualising variants using information on 3D chromosome conformation, now expanding to cover structural variation such as inversions, deletions, duplications, and insertions. A new database SomaMutDB ( 81 ) covers mutations—SNVs and small insertions or deletions—in somatic cells, linking them to data such as regulatory elements and gene expression data, to facilitate their analysis and comparison with much more common cancer-related mutation data. The publication from the European Genome-Phenome Archive ( 82 ), with its potentially identifiable genetic, phenotypic and clinical human data, coincides with an alteration to the guidelines for acceptance into the Database Issue (available online at https://academic.oup.com/nar/pages/ms_prep_database ). Previously, the Issue blanket disallowed any form of registration: henceforth such registration is allowed, but only in specific cases where it is legally required in order to protect the integrity of potentially identifiable human data. The EGA paper includes a detailed discussion of its access and download protocols, and of prospects for future sharing of such data.
The section on ‘ Human genomic variation, diseases and drugs ’ contains papers on two new resources for linking genetic variation to disease. VannoPortal ( 83 ) integrates no fewer than 40 data sources to provide impressively comprehensive linkages between variants and diseases or traits, and boasts a particularly clean and responsive interface. ConVarT ( 84 ) takes the approach of mapping equivalent variants between orthologous protein pairs between human and model organisms such as Caenorhabditis elegans . This allows experimental data on variant pathogenicity obtained from model organisms to help interpret the consequences of human variants. Molecules of the immune system are the focus of both the venerable IMGT ® databases which contributes an update ( 85 ), and the new human Antigen Receptor database (huARdb ( 86 )) which exploits new single-cell immune profiling and transcriptomics to reveal individual clonotypes of T-cell and B-cell receptors (TCRs and BCRs). Notably, huARdb offers stable URLs for results of analyses of user data at the site to facilitate interactive data sharing. Two further databases deal with antibodies, including nanobodies - antibodies consisting of a single monomeric variable domain. INDI ( 87 ) collects sequences and structures plus associated metadata from a variety of sources and allows various modes of sequence or text search. The authors envisage the dataset being valuable for computational efforts towards nanobody design. SAbDab focuses on antibody structures, updated weekly, and here describes increases in content along with a new SAbDab-nano section dedicated to nanobodies ( 88 ).
Elsewhere, drug combinations and interactions are covered by two new databases. DDInter ( 89 ) mines the literature for information on drug–drug interactions, classifying the results (synergy, antagonism etc.) and presenting interactions in a variety of attractive visualisations. NPCDR ( 90 ) works in a similar area but focuses on cases where at least one of the drugs involved is based on a natural product. Cellular responses to drugs are captured by the new CeDR database ( 91 ), which uses single cell transcriptomics data to capture the characteristic drug responses of different cells and tissues, in human and mouse and in health and disease. In a similar area, CTR-DB ( 92 ) contains clinical transcriptomics data from cancer patients, both pre-treatment and drug-induced. A myriad of analytical options maximise the data's value in, for example, biomarker discovery and understanding drug resistance mechanisms. Other new cancer-related databases include CancerMIRNome ( 93 ) that covers miRNAs in cancer cells and offers particularly rich analytical options; CancerSCEM ( 94 ) that offers similarly diverse options for studying single cancer cell gene expression data; GPEdit ( 95 ) which links A-to-I RNA editing in cancer cells to pharmacogenomic responses and patient survival; and OncoDB ( 96 ), which focuses on the contributions of gene expression dysregulation and viral infection to cancer development and progression. This year also sees Update papers from two major general resources in drug design. The IUPHAR/BPS guide to PHARMACOLOGY ( 97 ) reports on its efforts to curate information on drugs and drug targets for SARS-CoV-2, as well as updates to its sections on Malaria and antibacterials. The paper from the Therapeutic Target Database (TTD) ( 98 ) reports significant updates including many new kinds of data including information on weak or non-binders of targets, prodrug-drug pairs and AlphaFold models of drug targets for which experimental structures are not yet available. Finally, it's a pleasure to welcome the European Variation Archive (EVA) ( 99 ) to the Issue, a full eight years after its genesis. In that time its content has grown dramatically to now cover over 3 billion variants.
The ‘ Plant database ’ section includes an Update paper from the popular comparative genomics resource PLAZA ( 100 ) which reports a near-doubling of species covered and new and improved features throughout, including the API. The paper on BRAD ( 101 ), the dedicated Brassica database, reports a particular focus on synteny analysis tools and looks forward to accommodating the more diverse omics data and pangenome information now becoming available for the Family. Plant ncRNA is covered by returning databases GreeNC ( 102 ), with its focus on lncRNA, and PmiREN ( 103 ) which doubles its content of miRNA entries. The latter offers an impressive array of new features for functional and evolutionary exploration including gene regulatory elements, target annotations, variants and phylogenetic trees. Finally, welcome new arrivals include PlantGSAD ( 104 ) which provides >200 000 gene sets across 44 families, sets based on a notably diverse set of properties; and qPTMplants ( 105 ) which curates data, including quantitative information, on post-translational modifications (PTM) across 43 species. The latter features an interesting discussion of PTM crosstalk identified in the database.
The final ‘ Other databases ’ section includes Update papers from major proteomics resources. iProX, a member of the ProteomeXchange consortium ( 106 ) as now processed almost 100 TB of submitted data and reports new features such as an efficient reanalysis platform and an API ( 107 ). ProteomicsDB also reports a new API, generated with reference to FAIR principles ( 108 ), alongside a new interface with fresh visualisation options ( 109 ). An update from Proteome-p I ( 110 ) reports on a more than trebling of its content of predicted p I (isoelectric point) and p K a values for proteins and in silico digested peptides, parameters relevant to proteomics and other biophysical experiments. Finally, two new databases curate information previously only inconveniently scattered through the literature. dNTPpoolDB contains concentrations of deoxyribonucleotide triphosphates in different species, cells and experimental conditions ( 111 ) while ProNAB contains >20 000 data points on binding affinity of proteins (wild-type and mutant) for DNA or RNA ( 112 ).
NAR ONLINE MOLECULAR BIOLOGY DATABASE COLLECTION
We are pleased to include 1645 entries in this 29th release of the NAR online Molecular Database Collection (available at http://www.oxfordjournals.org/nar/database/c/ ). We have updated 317 entries, 89 new resources were added and 80 entries were removed in our ongoing effort to provide an up-to-date collection. We encourage authors to send their updates (in plain text according to the template found in http://www.oxfordjournals.org/nar/database/summary/1 ) to [email protected] .
ACKNOWLEDGEMENTS
We thank Dr Martine Bernardes-Silva, especially, and the rest of the Oxford University Press team led by Joanna Ventikos for their help in compiling this issue.
Contributor Information
Daniel J Rigden, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Crown Street, Liverpool L69 7ZB, UK.
Xosé M Fernández, Institut Curie, 25 rue d’Ulm, 75005 Paris, France.
Funding for open access charge: Oxford University Press.
Conflict of interest statement . The authors' opinions do not necessarily reflect the views of their respective institutions.
- 1. Cantelli G., Bateman A., Brooksbank C., Petrov A.I., Malik-Sheriff R.S., Ide-Smith M., Hermjakob H., Flicek P., Apweiler R., Birney E.et al.. The European Bioinformatics Institute (EMBL-EBI) in 2021. Nucleic Acids Res. 2021; 10.1093/nar/gkab1127. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Sayers E.W., Bolton E.E., Brister J.R., Canese K., Chan J., Comeau D.C., Connor R., Funk K., Kelly C., Kim S.et al.. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021; 10.1093/nar/gkab1112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. CNCB-NGDC Members and Partners Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2021; 10.1093/nar/gkab951. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Torrens-Fontanals M., Peralta-García A., Talarico C., Guixà-González R., Giorgino T., Selent J.. SCoV2-MD: a database for the dynamics of the SARS-CoV-2 proteome and variant impact predictions. Nucleic Acids Res. 2021; 10.1093/nar/gkab977. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. De Silva N.H., Bhai J., Chakiachvili M., Contreras-Moreira B., Cummins C., Frankish A., Gall A., Genez T., Howe K.L., Hunt S.E.et al.. The Ensembl COVID-19 resource: ongoing integration of public SARS-CoV-2 data. Nucleic Acids Res. 2021; 10.1093/nar/gkab889. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Kalvari I., Nawrocki E.P., Ontiveros-Palacios N., Argasinska J., Lamkiewicz K., Marz M., Griffiths-Jones S., Toffano-Nioche C., Gautheret D., Weinberg Z.et al.. Rfam 14: expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 2021; 49:D192–D200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Qi C., Wang C., Zhao L., Zhu Z., Wang P., Zhang S., Cheng L., Zhang X.. SCovid: single-cell atlases for exposing molecular characteristics of COVID-19 across 10 human tissues. Nucleic Acids Res. 2021; 10.1093/nar/gkab881. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Zhang W., Zhang Y., Min Z., Mo J., Ju Z., Guan W., Zeng B., Liu Y., Chen J., Zhang Q.et al.. COVID19db: a comprehensive database platform to discover potential drugs and targets of COVID-19 at whole transcriptomic scale. Nucleic Acids Res. 2021; 10.1093/nar/gkab850. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Nersisyan S., Zhiyanov A., Shkurnikov M., Tonevitsky A.. T-CoV: a comprehensive portal of HLA-peptide interactions affected by SARS-CoV-2 mutations. Nucleic Acids Res. 2021; 10.1093/nar/gkab701. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Rophina M., Pandhare K., Shamnath A., Imran M., Jolly B., Scaria V.. ESC: a comprehensive resource for SARS-CoV-2 immune escape variants. Nucleic Acids Res. 2021; 10.1093/nar/gkab895. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Sun Q., Shu C., Shi W., Luo Y., Fan G., Nie J., Bi Y., Wang Q., Qi J., Lu J.et al.. VarEPS: an evaluation and prewarning system of known and virtual variations of SARS-CoV-2 genomes. Nucleic Acids Res. 2021; 10.1093/nar/gkab921. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Yang X., Tong Y., Liu G., Yuan J., Yang Y.. scAPAatlas: an atlas of alternative polyadenylation across cell types in human and mouse. Nucleic Acids Res. 2021; 10.1093/nar/gkab917. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Zhu S., Lian Q., Ye W., Qin W., Wu Z., Ji G., Wu X.. scAPAdb: a comprehensive database of alternative polyadenylation at single-cell resolution. Nucleic Acids Res. 2021; 10.1093/nar/gkab795. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Gao T., Zheng Z., Pan Y., Zhu C., Wei F., Yuan J., Sun R., Fang S., Wang N., Zhou Y.et al.. scEnhancer: a single-cell enhancer resource with annotation across hundreds of tissue/cell types in three species. Nucleic Acids Res. 2021; 10.1093/nar/gkab1032. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Zong W., Kang H., Xiong Z., Ma Y., Jin T., Gong Z., Yi L., Zhang M., Wu S., Wang G.et al.. 2021) scMethBank: a database for single-cell whole genome DNA methylation maps. Nucleic Acids Res. 10.1093/nar/gkab833. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Li R., Liang F., Li M., Zou D., Sun S., Zhao Y., Zhao W., Bao Y., Xiao J., Zhang Z.. MethBank 3.0: a database of DNA methylomes across a variety of species. Nucleic. Acids. Res. 2018; 46:D288–D295. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Zhu H., Fu H., Cui T., Ning L., Shao H., Guo Y., Ke Y., Zheng J., Lin H., Wu X.et al.. RNAPhaSep: a resource of RNAs undergoing phase separation. Nucleic Acids Res. 2021; 10.1093/nar/gkab985. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Liu M., Li H., Luo X., Cai J., Chen T., Xie Y., Ren J., Zuo Z.. RPS: a comprehensive database of RNAs involved in liquid–liquid phase separation. Nucleic Acids Res. 2021; 10.1093/nar/gkab986. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Kang J., Tang Q., He J., Li L., Yang N., Yu S., Wang M., Zhang Y., Lin J., Cui T.et al.. RNAInter v4.0: RNA interactome repository with redefined confidence scoring system and improved accessibility. Nucleic Acids Res. 2021; 10.1093/nar/gkab997. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Cui T., Dou Y., Tan P., Ni Z., Liu T., Wang D., Huang Y., Cai K., Zhao X., Xu D.et al.. RNALocate v2.0: an updated resource for RNA subcellular localization with increased coverage and annotation. Nucleic Acids Res. 2021; 10.1093/nar/gkab825. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Castro-Mondragon J. A., Riudavets-Puig R., Rauluseviciute I., Berhanu Lemma R., Turchi L., Blanc-Mathieu R., Lucas J., Boddie P., Khan A., Manosalva Pérez N.et al.. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2021; 10.1093/nar/gkab1113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Pratt H.E., Andrews G.R., Phalke N., Purcaro M.J., van der Velde A., Moore J.E., Weng Z.. Factorbook: an updated catalog of transcription factor motifs and candidate regulatory motif sites. Nucleic Acids Res. 2021; 10.1093/nar/gkab1039. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Xu M., Bai X., Ai B., Zhang G., Song C., Zhao J., Wang Y., Wei L., Qian F., Li Y.et al.. TF-Marker: a comprehensive manually curated database for transcription factors and related markers in specific cell and tissue types in human. Nucleic Acids Res. 2021; 10.1093/nar/gkab1114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Zhang Y., Song C., Zhang Y., Wang Y., Feng C., Chen J., Wei L., Pan Q., Shang D., Zhu Y.et al.. TcoFBase: a comprehensive database for decoding the regulatory transcription co-factors in human and mouse. Nucleic Acids Res. 2021; 10.1093/nar/gkab950. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Boccaletto P., Stefaniak F., Ray A., Cappannini A., Mukherjee S., Purta E., Kurkowska M., Shirvanizadeh N., Destefanis E., Groza P.et al.. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2021; 10.1093/nar/gkab1083. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Huang H.-Y., Lin Y.-C.-D., Cui S., Huang Y., Tang Y., Xu J., Bao J., Li Y., Wen J., Zuo H.et al.. miRTarBase update 2022: an informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2021; 10.1093/nar/gkab1079. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Keller A., Gröger L., Tschernig T., Solomon J., Laham O., Schaum N., Wagner V., Kern F., Schmartz G.P., Li Y.et al.. miRNATissueAtlas2: an update to the human miRNA tissue atlas. Nucleic Acids Res. 2021; 10.1093/nar/gkab808. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Pereira J., Simpkin A.J., Hartmann M.D., Rigden D.J., Keegan R.M., Lupas A.N.. High-accuracy protein structure prediction in CASP14. Proteins Struct. Funct. Bioinf. 2021; 89:1687–1699. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., Bridgland A.. Highly accurate protein structure prediction with AlphaFold. Nature. 2021; 596:583–589. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Tunyasuvunakool K., Adler J., Wu Z., Green T., Zielinski M., Žídek A., Bridgland A., Cowie A., Meyer C., Laydon A.et al.. Highly accurate protein structure prediction for the human proteome. Nature. 2021; 596:590–596. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A.et al.. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021; 10.1093/nar/gkab1061. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Suzek B.E., Wang Y., Huang H., McGarvey P.B., Wu C.H.UniProt Consortium . UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics. 2015; 31:926–932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. The UniProt Consortium UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021; 49:D480–D489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Blum M., Chang H.Y., Chuguransky S., Grego T., Kandasaamy S., Mitchell A., Nuka G., Paysan-Lafosse T., Qureshi M., Raj S.et al.. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021; 49:D344–D354. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. PDBe-KB consortium PDBe-KB: collaboratively defining the biological context of structural data. Nucleic Acids Res. 2021; 10.1093/nar/gkab988. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Hollas M.A.R., Robey M.T., Fellers R.T., LeDuc R.D., Thomas P.M., Kelleher N.L.. The Human Proteoform Atlas: a FAIR community resource for experimentally derived proteoforms. Nucleic Acids Res. 2021; 10.1093/nar/gkab1086. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Pándy-Szekeres G., Esguerra M., Hauser A.S., Caroli J., Munk C., Pilger S., Keserű G.M., Kooistra A.J., Gloriam D.E.. The G protein database, GproteinDb. Nucleic Acids Res. 2021; 10.1093/nar/gkab852. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M.et al.. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021; 10.1093/nar/gkab1038. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Howe K.L., Achuthan P., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Azov A.G., Bennett R., Bhai J.et al.. Ensembl 2021. Nucleic Acids Res. 2021; 49:D884–D891. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Papatheodorou I., Moreno P., Manning J., Fuentes A.M., George N., Fexova S., Fonseca N.A., Füllgrabe A., Green M., Huang N.et al.. Expression Atlas update: from tissues to single cells. Nucleic Acids Res. 2020; 48:D77–D83. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Quaglia F., Mészáros B., Salladini E., Hatos A., Pancsa R., Chemes L.B., Pajkos M., Lazar T., Peña-Díaz S., Santos J.et al.. DisProt in 2022: improved quality and accessibility of protein intrinsic disorder annotation. Nucleic Acids Res. 2021; 10.1093/nar/gkab1082. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Hatos A., Quaglia F., Piovesan D., Tosatto S.C.. APICURON: a database to credit and acknowledge the work of biocurators. Database. 2021; 2021:baab019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Hatos A., Monzon A.M., Tosatto S.C.E., Piovesan D., Fuxreiter M.. FuzDB: a new phase in understanding fuzzy interactions. Nucleic Acids Res. 2021; 10.1093/nar/gkab1060. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Kumar M., Michael S., Alvarado-Valverde J., Mészáros B., Sámano-Sánchez H., Zeke A., Dobson L., Lazar T., Örd M., Nagpal A.et al.. The Eukaryotic Linear Motif resource: 2022 release. Nucleic Acids Res. 2021; 10.1093/nar/gkab975. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Kanehisa M., Furumichi M., Sato Y., Ishiguro-Watanabe M., Tanabe M.. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021; 49:D545–D551. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Gillespie M., Jassal B., Stephan R., Milacic M., Rothfels K., Senff-Ribeiro A., Griss J., Sevilla C., Matthews L., Gong C.et al.. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2021; 10.1093/nar/gkab1028. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Oprea T.I., Bologa C.G., Brunak S., Campbell A., Gan G.N., Gaulton A., Gomez S.M., Guha R., Hersey A., Holmes J.et al.. Unexplored therapeutic opportunities in the human genome. Nat. Rev. Drug Discovery. 2018; 17:317–332. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Kamburov A., Herwig R.. ConsensusPathDB 2022: molecular interactions update as a resource for network biology. Nucleic Acids Res. 2021; 10.1093/nar/gkab1128. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Modi V., Dunbrack R.L. Jr. Kincore: a web resource for structural classification of protein kinases and their inhibitors. Nucleic Acids Res. 2021; 10.1093/nar/gkab920. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Wishart D.S., Guo A., Oler E., Wang F., Anjum A., Peters H., Dizon R., Sayeeda Z., Tian S., Lee B.L.et al.. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 2021; 10.1093/nar/gkab1062. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Drula E., Garron M.-L., Dogan S., Lombard V., Henrissat B., Terrapon N.. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 2021; 10.1093/nar/gkab1045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Ondov B.D., Bergman N.H., Phillippy A.M.. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics. 2011; 12:1–10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. del Toro N., Shrivastava A., Ragueneau E., Meldal B., Combe C., Barrera E., Perfetto L., How K., Ratan P., Shirodkar G.et al.. The IntAct database: efficient access to fine-grained molecular interaction data. Nucleic Acids Res. 2021; 10.1093/nar/gkab1006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Meldal B.H.M., Perfetto L., Combe C., Lubiana T., Cavalcante J.V.F., Bye-A-Jee H., Waagmeester A., del-Toro N., Shrivastava A., Barrera E.et al.. Complex Portal 2022: new curation frontiers. Nucleic Acids Res. 2021; 10.1093/nar/gkab991. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Meier-Kolthoff J.P., Carbasse J.S., Peinado-Olarte R.L., Göker M.. TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2021; 10.1093/nar/gkab902. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Parks D.H., Chuvochina M., Rinke C., Mussig A.J., Chaumeil P.-A., Hugenholtz P.. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2021; 10.1093/nar/gkab776. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Arita M., Karsch-Mizrachi I., Cochrane G.. The international nucleotide sequence database collaboration. Nucleic Acids Res. 2021; 49:D121–D124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Mitchell A.L., Almeida A., Beracochea M., Boland M., Burgin J., Cochrane G., Crusoe M.R., Kale V., Potter S.C., Richardson L.J.et al.. MGnify: the microbiome analysis resource in 2020. Nucleic Acids Res. 2020; 48:D570–D578. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Jin H., Hu G., Sun C., Duan Y., Zhang Z., Liu Z., Zhao X.-M., Chen W.-H.. mBodyMap: a curated database for microbes across human body and their associations with health and diseases. Nucleic Acids Res. 2021; 10.1093/nar/gkab973. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Cheng L., Qi C., Yang H., Lu M., Cai Y., Fu T., Ren J., Jin Q., Zhang X.. gutMGene: a comprehensive database for target genes of gut microbes and microbial metabolites. Nucleic Acids Res. 2021; 10.1093/nar/gkab786. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Yang J., Park J., Jung Y., Chun J.. AMDB: a database of animal gut microbial communities with manually curated metadata. Nucleic Acids Res. 2021; 10.1093/nar/gkab1009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Urban M., Cuzick A., Seager J., Wood V., Rutherford K., Venkatesh S.Y., Sahu J., Iyer S.V., Khamari L., De Silva N.et al.. PHI-base in 2022: a multi-species phenotype database for Pathogen–Host Interactions. Nucleic Acids Res. 2021; 10.1093/nar/gkab1037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Amos B., Aurrecoechea C., Barba M., Barreto A., Basenko E.Y., Bażant W., Belnap R., Blevins A.S., Böhme U., Brestelli J.et al.. VEuPathDB: the eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 2021; 10.1093/nar/gkab929. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Giraldo-Calderón G.I., Emrich S.J., MacCallum R.M., Maslen G., Dialynas E., Topalis P., Ho N., Gesing S., Madey G.VectorBase Consortium et al.. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015; 43:D707–D713. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Aurrecoechea C., Barreto A., Basenko E.Y., Brestelli J., Brunk B.P., Cade S., Crouch K., Doherty R., Falke D., Fischer S.et al.. EuPathDB: the eukaryotic pathogen genomics database resource. Nucleic Acids Res. 2017; 45:D581–D591. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Liu B., Zheng D., Zhou S., Chen L., Yang J.. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2021; 10.1093/nar/gkab1107. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Chen D., Tan C., Ding P., Luo L., Zhu J., Jiang X., Ou Z., Ding X., Lan T., Zhu Y.et al.. VThunter: a database for single-cell screening of virus target cells in the animal kingdom. Nucleic Acids Res. 2021; 10.1093/nar/gkab894. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Zhou S., Liu B., Han Y., Wang Y., Chen L., Wu Z., Yang J.. ZOVER: the database of zoonotic and vector-borne viruses. Nucleic Acids Res. 2021; 10.1093/nar/gkab862. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Cunningham F., Allen J.E., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Austine-Orimoloye O., Azov A.G., Barnes I., Bennett R.et al.. Ensembl 2022. Nucleic Acids Res. 2021; 10.1093/nar/gkab1049. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Yates A.D., Allen J., Amode R.M., Azov A.G., Barba M., Becerra A., Bhai J., Campbell L.I., Martinez M.C., Chakiachvili M.et al.. Ensembl Genomes 2022: an expanding genome resource for non-vertebrates. Nucleic Acids Res. 2021; 10.1093/nar/gkab1007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Lee B.T., Barber G.P., Benet-Pagès A., Casper J., Clawson H., Diekhans M., Fischer C., Gonzalez J.N., Hinrichs A.S., Lee C.M.et al.. The UCSC Genome Browser database: 2022 update. Nucleic Acids Res. 2021; 10.1093/nar/gkab959. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Fu W., Wang R., Nanaei H.A., Wang J., Hu D., Jiang Y.. 2021) RGD v2.0: a major update of the ruminant functional and evolutionary genomics database. Nucleic Acids Res. 10.1093/nar/gkab887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Mei Y., Jing D., Tang S., Chen X., Chen H., Duanmu H., Cong Y., Chen M., Ye X., Zhou H.et al.. InsectBase 2.0: a comprehensive gene resource for insects. Nucleic Acids Res. 2021; 10.1093/nar/gkab1090. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Walsh A.T., Triant D.A., Le Tourneau J.J., Shamimuzzaman M., Elsik C.G.. Hymenoptera Genome Database: new genomes and annotation datasets for improved go enrichment and orthologue analyses. Nucleic Acids Res. 2021; 10.1093/nar/gkab1018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. The Gene Ontology Consortium The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021; 49:D325–D334. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Krause S.A., Overend G., Dow J.A.T., Leader D.P.. FlyAtlas 2 in 2022: enhancements to the Drosophila melanogaster expression atlas. Nucleic Acids Res. 2021; 10.1093/nar/gkab971. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Colomer-Vilaplana A., Murga-Moreno J., Canalda-Baltrons A., Inserte C., Soto D., Coronado-Zamora M., Barbadilla A., Casillas S.. PopHumanVar: an interactive application for the functional characterization and prioritization of adaptive genomic variants in humans. Nucleic Acids Res. 2021; 10.1093/nar/gkab925. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Casillas S., Mulet R., Villegas-Mirón P., Hervas S., Sanz E., Velasco D., Bertranpetit J., Laayouni H., Barbadilla A.. PopHuman: the human population genomics browser. Nucleic Acids Res. 2018; 46:D1003–D1010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Colomer-Vilaplana A., Murga-Moreno J., Canalda-Baltrons A., Inserte C., Soto D., Coronado-Zamora M., Barbadilla A., Casillas S.. PopHumanVar: an interactive application for the functional characterization and prioritization of adaptive genomic variants in humans. Nucleic Acids Res. 2021; 10.1093/nar/gkab925. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Quan C., Ping J., Lu H., Zhou G., Lu Y.. 3DSNP 2.0: update and expansion of the noncoding genomic variant annotation database. Nucleic Acids Res. 2021; 10.1093/nar/gkab1008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Sun S., Wang Y., Maslov A.Y., Dong X., Vijg J.. SomaMutDB: a database of somatic mutations in normal human tissues. Nucleic Acids Res. 2021; 10.1093/nar/gkab914. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Freeberg M.A., Fromont L.A., D’Altri T., Romero A.F., Ciges J.I., Jene A., Kerry G., Moldes M., Ariosa R., Bahena S.et al.. The European Genome-phenome Archive in 2021. Nucleic Acids Res. 2021; 10.1093/nar/gkab1059. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Huang D., Zhou Y., Yi X., Fan X., Wang J., Yao H., Sham P.C., Hao J., Chen K., Li M.J.. VannoPortal: multiscale functional annotation of human genetic variants for interrogating molecular mechanism of traits and diseases. Nucleic Acids Res. 2021; 10.1093/nar/gkab853. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Pir M.S., Bilgin H.I., Sayici A., Coşkun F., Torun F.M., Zhao P., Kang Y., Cevik S., Kaplan O.I.. ConVarT: a search engine for matching human genetic variants with variants from non-human species. Nucleic Acids Res. 2021; 10.1093/nar/gkab939. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Manso T., Folch G., Giudicelli V., Jabado-Michaloud J., Kushwaha A., Nguefack Ngoune V., Georga M., Papadaki A., Debbagh C., Pégorier P.et al.. 2021) IMGT ® databases, related tools and web resources through three main axes of research and development. Nucleic Acids Res. 10.1093/nar/gkab1136. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Wu L., Xue Z., Jin S., Zhang J., Guo Y., Bai Y., Jin X., Wang C., Wang L., Liu Z., Wang J.Q.et al.. 2021) huARdb: human Antigen Receptor database for interactive clonotype-transcriptome analysis at the single-cell level. Nucleic Acids Res. 10.1093/nar/gkab857. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Deszyński P., Młokosiewicz J., Volanakis A., Jaszczyszyn I., Castellana N., Bonissone S., Ganesan R., Krawczyk K.. INDI—integrated nanobody database for immunoinformatics. Nucleic Acids Res. 2021; 10.1093/nar/gkab1021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Schneider C., Raybould M.I.J., Deane C.M.. SAbDab in the age of biotherapeutics: updates including SAbDab-nano, the nanobody structure tracker. Nucleic Acids Res. 2021; 10.1093/nar/gkab1050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Xiong G., Yang Z., Yi J., Wang N., Wang L., Zhu H., Wu C., Lu A., Chen X., Liu S.et al.. DDInter: an online drug–drug interaction database towards improving clinical decision-making and patient safety. Nucleic Acids Res. 2021; 10.1093/nar/gkab880. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Sun X., Zhang Y., Zhou Y., Lian X., Yan L., Pan T., Jin T., Xie H., Liang Z., Qiu W.et al.. Nucleic Acids Res. 2021; 10.1093/nar/gkab913. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Wang Y.-Y., Kang H., Xu T., Hao L., Bao Y., Jia P.. CeDR Atlas: a knowledgebase of cellular drug response. Nucleic Acids Res. 2021; 10.1093/nar/gkab897. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Liu Z., Liu J., Liu X., Wang X., Xie Q., Zhang X., Kong X., He M., Yang Y., Deng X.et al.. CTR-DB, an omnibus for patient-derived gene expression signatures correlated with cancer drug response. Nucleic Acids Res. 2021; 10.1093/nar/gkab860. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Li R., Qu H., Wang S., Chater J.M., Wang X., Cui Y., Yu L., Zhou R., Jia Q., Traband R.et al.. CancerMIRNome: an interactive analysis and visualization database for miRNome profiles of human cancer. Nucleic Acids Res. 2021; 10.1093/nar/gkab784. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Zeng J., Zhang Y., Shang Y., Mai J., Shi S., Lu M., Bu C., Zhang Z., Zhang Z., Li Y.et al.. CancerSCEM: a database of single-cell expression map across various human cancers. Nucleic Acids Res. 2021; 10.1093/nar/gkab905. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Ruan H., Li Q., Liu Y., Liu Y., Lussier C., Diao L., Han L.. GPEdit: the genetic and pharmacogenomic landscape of A-to-I RNA editing in cancers. Nucleic Acids Res. 2021; 10.1093/nar/gkab810. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Tang G., Cho M., Wang X.. OncoDB: an interactive online database for analysis of gene expression and viral infection in cancer. Nucleic Acids Res. 2021; 10.1093/nar/gkab970. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Harding S.D., Armstrong J.F., Faccenda E., Southan C., Alexander S.P.H., Davenport A.P., Pawson A.J., Spedding M., Davies J.A.NC-IUPHAR . The IUPHAR/BPS guide to PHARMACOLOGY in 2022: curating pharmacology for COVID-19, malaria and antibacterials. Nucleic Acids Res. 2021; 10.1093/nar/gkab1010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Zhou Y., Zhang Y., Lian X., Li F., Wang C., Zhu F., Qiu Y., Chen Y.. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2021; 10.1093/nar/gkab953. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Cezard T., Cunningham F., Hunt S.E., Koylass B., Kumar N., Saunders G., Shen A., Silva A.F., Tsukanov K., Venkataraman S.et al.. The European Variation Archive: a FAIR resource of genomic variation for all species. Nucleic Acids Res. 2021; 10.1093/nar/gkab960. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Van Bel M., Silvestri F., Weitz E.M., Kreft L., Botzki A., Coppens F., Vandepoele K.. PLAZA 5.0: extending the scope and power of comparative and functional genomics in plants. Nucleic Acids Res. 2021; 10.1093/nar/gkab1024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Chen H., Wang T., He X., Cai X., Lin R., Liang J., Wu J., King G., Wang X.. BRAD V3.0: an upgraded Brassicaceae database. Nucleic Acids Res. 2021; 10.1093/nar/gkab1057. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Di Marsico M., Gallart A.P., Sanseverino W., Cigliano R.A.. GreeNC 2.0: a comprehensive database of plant long non-coding RNAs. Nucleic Acids Res. 2021; 10.1093/nar/gkab1014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Guo Z., Kuang Z., Zhao Y., Deng Y., He H., Wan M., Tao Y., Wang D., Wei J., Li L.et al.. PmiREN2.0: from data annotation to functional exploration of plant microRNAs. Nucleic Acids Res. 2021; 10.1093/nar/gkab811. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Ma X., Yan H., Yang J., Liu Y., Li Z., Sheng M., Cao Y., Yu X., Yi X., Xu W.et al.. PlantGSAD: a comprehensive gene set annotation database for plant species. Nucleic Acids Res. 2021; 10.1093/nar/gkab794. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Xue H., Zhang Q., Wang P., Cao B., Jia C., Cheng B., Shi Y., Guo W.-F., Wang Z., Liu Z.-X.et al.. qPTMplants: an integrative database of quantitative post-translational modifications in plants. Nucleic Acids Res. 2021; 10.1093/nar/gkab945. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Deutsch E.W., Bandeira N., Sharma V., Perez-Riverol Y., Carver J.J., Kundu D.J., García-Seisdedos D., Jarnuczak A.F., Hewapathirana S., Pullman B.S.et al.. The ProteomeXchange consortium in 2020: enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020; 48:D1145–D1152. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Chen T., Ma J., Liu Y., Chen Z., Xiao N., Lu Y., Fu Y., Yang C., Li M., Wu S.et al.. iProX in 2021: connecting proteomics data sharing with big data. Nucleic. Acids. Res. 2021; 10.1093/nar/gkab1081. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Wilkinson M.D., Dumontier M., Aalbersberg I.J., Appleton G., Axton M., Baak A., Blomberg N., Boiten J.W., da Silva Santos L.B., Bourne P.E.et al.. The FAIR Guiding Principles for scientific data management and stewardship. Scientific data. 2016; 3:1–9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Lautenbacher L., Samaras P., Muller J., Grafberger A., Shraideh M., Rank J., Fuchs S.T., Schmidt T.K., The M., Dallago C.et al.. ProteomicsDB: toward a FAIR open-source resource for life-science research. Nucleic Acids Res. 2021; 10.1093/nar/gkab1026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Kozlowski L.P. Proteome- pI 2.0: proteome isoelectric point database update. Nucleic Acids Res. 2021; 10.1093/nar/gkab944. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Pancsa R., Fichó E., Molnár D., Surányi É.V., Trombitás T., Füzesi D., Lóczi H., Szijjártó P., Hirmondó R., Szabó J.E.et al.. dNTPpoolDB: a manually curated database of experimentally determined dNTP pools and pool changes in biological samples. Nucleic Acids Res. 2021; 10.1093/nar/gkab910. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Harini K., Srivastava A., Kulandaisamy A., Gromiha M.M.. ProNAB: database for binding affinities of protein–nucleic acid complexes and their mutants. Nucleic Acids Res. 2021; 10.1093/nar/gkab848. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (189.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Search Menu
- Sign in through your institution
- Chemical Biology and Nucleic Acid Chemistry
- Computational Biology
- Critical Reviews and Perspectives
- Data Resources and Analyses
- Gene Regulation, Chromatin and Epigenetics
- Genome Integrity, Repair and Replication
- Molecular and Structural Biology
- Nucleic Acid Enzymes
- Nucleic Acid Therapeutics
- RNA and RNA-protein complexes
- Synthetic Biology and Bioengineering
- Advance Articles
- Breakthrough Articles
- Molecular Biology Database Collection
- Special Collections
- Scope and Criteria for Consideration
- Author Guidelines
- Data Deposition Policy
- Database Issue Guidelines
- Web Server Issue Guidelines
- Submission Site
- Open Access Charges
- About Nucleic Acids Research
- Editors & Editorial Board
- Information of Referees
- Self-Archiving Policy
- Dispatch Dates
- Advertising and Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic
Advance articles
Correction to ‘ncbi taxonomy: enhanced access via ncbi datasets’.
- View article
Structural insights into a DNA polymerase reading the xeno nucleic acid HNA

- Supplementary data
Programming ADAR-recruiting hairpin RNA sensor to detect endogenous molecules

RPA and Rad27 limit templated and inverted insertions at DNA breaks

Point mutations in functionally diverse genes are associated with increased natural DNA transformation in multidrug resistant Streptococcus pneumoniae

Enzymatic bypass of G-quadruplex structures containing oxidative lesions

Repeated fasting events sensitize enhancers, transcription factor activity and gene expression to support augmented ketogenesis

Novel role of zinc-finger protein 518 in heterochromatin formation on α-satellite DNA

KRBP72 facilitates ATPase-dependent editing progression through a structural roadblock in mitochondrial A6 mRNA

CRISPR-based dissection of miRNA binding sites using isogenic cell lines is hampered by pervasive noise

Immunosenescence Inventory—a multi-omics database for immune aging research

Adaptive immunity of type VI CRISPR-Cas systems associated with reverse transcriptase–Cas1 fusion proteins

MirGeneDB 3.0: improved taxonomic sampling, uniform nomenclature of novel conserved microRNA families and updated covariance models

Molecular basis for thiocarboxylation and release of Urm1 by its E1-activating enzyme Uba4

Functional RNA mining using random high-throughput screening

Optimization of ACE-tRNAs function in translation for suppression of nonsense mutations

Distinct interactomes of ADAR1 nuclear and cytoplasmic protein isoforms and their responses to interferon induction

Molecular basis of the phosphorothioation-sensing antiphage defense system IscS–DndBCDE–DndI

Control of iron acquisition by multiple small RNAs unravels a new role for transcriptional terminator loops in gene regulation

CZ CELLxGENE Discover: a single-cell data platform for scalable exploration, analysis and modeling of aggregated data

Updated resources for exploring experimentally-determined PDB structures and Computed Structure Models at the RCSB Protein Data Bank

Utilizing insights of DNA repair machinery to discover MMEJ deletions and novel mechanisms

EMBL’s European Bioinformatics Institute (EMBL-EBI) in 2024

Optogenetic control of Corynebacterium glutamicum gene expression

Tetramerization of deoxyadenosine kinase meets the demands of a DNA replication substrate challenge in Giardia intestinalis

DNA sequence and lesion-dependent mitochondrial transcription factor A (TFAM)-DNA-binding modulates DNA repair activities and products

UHRF1 ubiquitin ligase activity supports the maintenance of low-density CpG methylation

Chromatin and transcription in Nucleic Acids Research: the first 50 years
Update of the fantom web resource: enhancement for studying noncoding genomes.


PHI-base – the multi-species pathogen–host interaction database in 2025

COCONUT 2.0: a comprehensive overhaul and curation of the collection of open natural products database

REDIportal: toward an integrated view of the A-to-I editing

High-throughput capture of transcription factor-driven epigenome dynamics using PHILO ChIP-seq

Chemotherapeutic agents and leucine deprivation induce codon-biased aberrant protein production in cancer

Bacterial Rps3 counters oxidative and UV stress by recognizing and processing AP-sites on mRNA via a novel mechanism

Phosphorylation-mediated disassembly of C-terminal binding protein 2 tetramer impedes epigenetic silencing of pluripotency in mouse embryonic stem cells

Interpreting CRISPR-Cas12a enzyme kinetics through free energy change of nucleic acids

Circular oligomeric particles formed by Ros/MucR family members mediate DNA organization in α-proteobacteria

Bacterial WYL domain transcriptional repressors sense single-stranded DNA to control gene expression
Retargeting target-directed microRNA-decay sites to highly expressed viral or cellular miRNAs

STK39-mediated amplification of γ-H2A.X promotes homologous recombination and contributes to PARP inhibitor resistance

The Natural Products Atlas 3.0: extending the database of microbially derived natural products

GoFCards: an integrated database and analytic platform for gain of function variants in humans

ClinVar: updates to support classifications of both germline and somatic variants

miRStart 2.0: enhancing miRNA regulatory insights through deep learning-based TSS identification

miRTarBase 2025: updates to the collection of experimentally validated microRNA–target interactions

The PIWI-interacting protein Gtsf1 controls the selective degradation of small RNAs in Paramecium

- Lay summary
BFVD—a large repository of predicted viral protein structures

Simultaneous determination of cytosolic aminoacyl-tRNA synthetase activities by LC–MS/MS

BindingDB in 2024: a FAIR knowledgebase of protein-small molecule binding data

The Natural Products Magnetic Resonance Database (NP-MRD) for 2025

PanKB: An interactive microbial pangenome knowledgebase for research, biotechnological innovation, and knowledge mining

Identifying key underlying regulatory networks and predicting targets of orphan C/D box SNORD116 snoRNAs in Prader–Willi syndrome

Correction to ‘Comparative Toxicogenomics Database's 20th anniversary: update 2025’
Deep learning insights into distinct patterns of polygenic adaptation across human populations.

Dimerization of the deaminase domain and locking interactions with Cas9 boost base editing efficiency in ABE8e

Single-stranded DNA with internal base modifications mediates highly efficient knock-in in primary cells using CRISPR-Cas9

GENCODE 2025: reference gene annotation for human and mouse

InterPro: the protein sequence classification resource in 2025

sc2GWAS: a comprehensive platform linking single cell and GWAS traits of human

PGxDB: an interactive web-platform for pharmacogenomics research

CATH v4.4: major expansion of CATH by experimental and predicted structural data

PWAS Hub: exploring gene-based associations of complex diseases with sex dependency

Interrogation of the interplay between DNA N 6 -methyladenosine (6mA) and hypoxia-induced chromatin accessibility by a randomized empirical model (EnrichShuf)

Protein moonlighting by a target gene dominates phenotypic divergence of the Sef1 transcriptional regulatory network in yeasts

[SNG2], a prion form of Cut4/Apc1, confers non-Mendelian inheritance of heterochromatin silencing defect in fission yeast

A systematic quantitative approach comprehensively defines domain-specific functional pathways linked to Schizosaccharomyces pombe heterochromatin regulation

L1-ORF1p nucleoprotein can rapidly assume distinct conformations and simultaneously bind more than one nucleic acid

ECOD: integrating classifications of protein domains from experimental and predicted structures

The PLSDB 2025 update: enhanced annotations and improved functionality for comprehensive plasmid research

Glucose binds and activates NSUN2 to promote translation and epidermal differentiation

Crosstalk between paralogs and isoforms influences p63-dependent regulatory element activity

Harmonizome 3.0: integrated knowledge about genes and proteins from diverse multi-omics resources

Profiling of i-motif-binding proteins reveals functional roles of nucleolin in regulation of high-order DNA structures

RADIP technology comprehensively identifies H3K27me3-associated RNA–chromatin interactions

Functional and molecular insights into the role of Sae2 C-terminus in the activation of MRX endonuclease

GenBank 2025 update

CAUSALdb2: an updated database for causal variants of complex traits

Efficient suppression of premature termination codons with alanine by engineered chimeric pyrrolysine tRNAs

The European Nucleotide Archive in 2024

OncoSplicing 3.0: an updated database for identifying RBPs regulating alternative splicing events in cancers

Full-length direct RNA sequencing reveals extensive remodeling of RNA expression, processing and modification in aging Caenorhabditis elegans

scCancerExplorer: a comprehensive database for interactively exploring single-cell multi-omics data of human pan-cancer

NASA open science data repository: open science for life in space

Plant Metabolic Network 16: expansion of underrepresented plant groups and experimentally supported enzyme data

Intrinsically disordered RNA-binding motifs cooperate to catalyze RNA folding and drive phase separation

AcrIIIA1 is a protein–RNA anti-CRISPR complex that targets core Cas and accessory nucleases

UniProt: the Universal Protein Knowledgebase in 2025

HERB 2.0: an updated database integrating clinical and experimental evidence for traditional Chinese medicine

GPCRdb in 2025: adding odorant receptors, data mapper, structure similarity search and models of physiological ligand complexes

The Chemical Probes Portal – 2024: update on this public resource to support best-practice selection and use of small molecules in biomedical research

CAF-1 promotes efficient PrimPol recruitment to nascent DNA for single-stranded DNA gap formation

PubChem 2025 update

Complex portal 2025: predicted human complexes and enhanced visualisation tools for the comparison of orthologous and paralogous complexes

The rice genome annotation project: an updated database for mining the rice genome

The STRING database in 2025: protein networks with directionality of regulation

MatrixDB 2024: an increased coverage of extracellular matrix interactions, a new Network Explorer and a new web interface

A deep mutational scanning platform to characterize the fitness landscape of anti-CRISPR proteins

The Immune Epitope Database (IEDB): 2024 update

Selective recognition of RNA G-quadruplex in vitro and in cells by L-aptamer–D-oligonucleotide conjugate

Email alerts
- Editorial Board
- X (formerly Twitter)
Affiliations
- Online ISSN 1362-4962
- Print ISSN 0305-1048
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
The 2024 Nucleic Acids Research Database issue contains 180 papers, including 90 papers reporting on new databases and 83 updates from resources previously published in the issue. Seven additional manuscripts provide updates on databases most recently published elsewhere.
Nucleic Acids Research (NAR) publishes the results of leading-edge research into physical, chemical, biochemical and biological aspects of nucleic acids and proteins involved in nucleic acid metabolism and/or interactions. It enables the rapid publication of papers under the following categories:
Nucleic Acids Research, Volume 52, Issue 21, 27 November 2024, Pages 12767–12783, https://doi.org/10.1093/nar/gkae787 Abstracts Lay summary
Nucleic Acids Research is an open-access journal on nucleic acids and related topics, published by Oxford University Press since 1974. It has a high impact factor and publishes special issues on biological databases and web servers.
Over the decades, Nucleic Acids Research has published and promoted many studies in areas that have fundamentally transformed molecular biology and biomedical research, including the biological role, development and application of restriction endonucleases and CRISPR-based gene targeting systems , the characterization of complex DNA and RNA ...
Nucleic acids (DNA or RNA) are polymers of nucleotides – each nucleotide consists of a pentose sugar, a phosphate group and one of the nitrogenous bases (purines and pyrimidines).
Nucleic acid drugs (NADs) are a new generation of gene-editing modalities characterized by their high efficiency and rapid development, which have become an active research topic in new drug ...
Nucleic acid sensing is an essential mechanism in tumor immunotherapy and gene therapies that target cancer and infectious diseases through genetically engineered immune cells or therapeutic ...
The 2022 Nucleic Acids Research Database Issue contains 185 papers, including 87 papers reporting on new databases and 85 updates from resources previously published in the Issue. Thirteen additional manuscripts provide updates on databases most recently published elsewhere.
Nucleic Acids Research, gkae1014, https://doi.org/10.1093/nar/gkae1014 Published: 4 November 2024 Section: STRUCTURAL BIOLOGY Abstracts