- Case report
- Open access
- Published: 07 October 2013

A young traveller presenting with typhoid fever after oral vaccination: a case report
- Martin Grimm 1 , 2 ,
- Christoph Lübbert 2 ,
- Joachim Mössner 2 &
- Sebastian Weis 2
Journal of Medical Case Reports volume 7 , Article number: 237 ( 2013 ) Cite this article
24k Accesses
2 Citations
10 Altmetric
Metrics details
Introduction
Typhoid fever is one of the most common vaccine-preventable diseases in travellers returning from tropical destinations. However, immunity and the immune response to infection are barely understood.
Case presentation
We report a case of tyhoid fever in a 29-year-old Caucasian, previously healthy woman who did not develop protective immunity or seroconversion of H or O antibodies neither after vaccination with the oral Ty21 vaccine, nor after infection with Salmonella typhi .
Conclusions
This case highlights the insufficiencies of the current vaccination and the lack of a reliable, rapid serologic diagnostic tool for typhoid fever. With this case report, we aim to sensitize the reader that typhoid fever has to be taken into account as a differential diagnosis in patients even after vaccination and with negative serological test results.
Peer Review reports
Enteric fever caused by Salmonella enterica serovar typhi is the most common bacteraemic and vaccine-preventable disease in travellers returning from the tropics [ 1 , 2 ]. It is a rare diagnosis in the western world. In Germany for example, there were less than 80 cases annually during the last few years [ 3 ]. Nevertheless, typhoid fever has to be considered as a differential diagnosis especially in patients with a travel history to high-endemic areas such as India. Prevention and diagnosis of typhoid fever are hampered as immunity and the immune response to infection are barely understood.
A 29-year-old female German law student was referred to our outpatient department (OPD) with a two-week history of severe frontal headache and high-grade fever reaching 41°C (106°F). Upon her first presentation, diarrhea, bloody discharge or abdominal cramps were denied. She did not report any weight changes or high-risk sexual behaviour. She had no previous history of diseases and was not on any medication. Paracetamol had relieved pain and fever for up to eight hours.
Her travel history was remarkable for a three-month sojourn to Delhi, India, where she had completed an internship in an upper-class neighbourhood and from which she had returned three weeks prior to presentation. During the last months, she had not taken any antibiotics and was not on malaria prophylaxis. Before travelling, she had received all recommended vaccinations including for typhoid fever (Ty21a) and hepatitis A. Her clinical examination was unremarkable. Routine laboratory tests showed a mild thrombocytopenia of 112Gpt/L (150 to 300) whilst leukocyte counts were not elevated. Her rapid malaria test results were negative. Serum antibodies against typhoid fever (O and H antigens, cutoff 1:200), Serum antibodies against typhoid fever (O and H antigens, cutoff 1:200), human immunodeficiency virus (HIV), herpes simplex virus (HSV) 1/2, dengue fever, and hepatitis C could not be detected. Stool microscopy, stool culture and specific antigene assays were unremarkable for pathologic bacteria, viral antigens, worms or protozoa. As she had no fever at the time of presentation, the attending physician did not take blood cultures.
However, she returned to the OPD five days later as her fever and headaches had not ceased. She then also complained of a non-productive cough, bone and muscle pain, abdominal discomfort and constipation. Blood cultures were taken and she was admitted to the gastroenterology ward.
On clinical examination, we saw a deterioration in the young woman’s general and nutritional condition. Her body temperature was 38.3°C (101°F); her heart rate was 90/min with a blood pressure of 115/70mmHg. There was no lymph node swelling. Her sclera were white and without suffusions. Her chest examination result was unremarkable. Her abdomen was slightly tense with reduced bowel sounds. There was no liver or spleen enlargement. Her laboratory test results were remarkable for elevated C-reactive protein (CRP) 142mg/L (<5); aspartate aminotransferase (ASAT) 8μkat/L; alanine aminotransferase (ALAT) 5μkat/L (both <0.6); lactate dehydrogenase (LDH) 17μkat/L (2.2 to 3.5); a decreased thrombocyte count of 109/μL and a cholinesterase activity of 58μkat/L (71 to 181). There was no eosinophilia, and microscopic differentiation showed normal leucocyte values (4.8Gpt/L (4.0 to 9.0), neutrophils 66.6%, lymphocytes 27.5%, monocytes 5.5%, eosinophils 0.0%, basophils 0.4%). Her chest X-ray revealed milky opacities of the basal parts of the lung as well as a prominent scoliosis (Figure 1 ). A urinary tract infection was ruled out. Stool microscopy and culture were again negative. A calculated antibiotic regimen with intravenous ciprofloxacin 500mg twice daily was initiated and switched to an oral formula after three days. Her antibody test results (including the Widal test) were again negative.
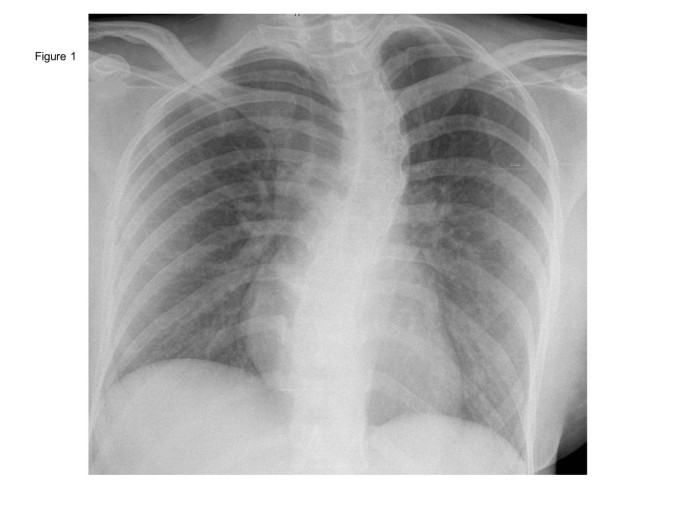
Chest X-ray taken at the first presentation showing milky opacifications in the basal parts of both lungs as well as a scoliosis. Courtesy of Prof. Kahn, Department of Imaging, University Hospital Leipzig.
Five days later, three out of four blood cultures returned positive for Salmonella enterica serovar typhi . The pathogen was found to be sensible to all tested antibiotics including beta-lactams, fluoroquinolones and cotrimoxazole. Chloramphenicol sensibility was not tested. On the same day, the fever decreased and the headaches resolved. Repeated stool controls were negative for Salmonella spp . The patient was discharged after seven days in an improved condition. In order to control Salmonella carriage further, stool controls were performed by German health authorities. Only one out of six stool samples turned out to be positive for Salmonella typhi . Up to now, the patient remains healthy and has not experienced any typhoid relapse.
Despite being rarely seen in western world hospitals, infection with S. typhi remains a global health issue and an important differential diagnosis in patients the return from tropical destinations. The World Health Organization (WHO) estimated 22 million cases and 200,000 deaths per year worldwide [ 4 ].
Typhoid fever can be prevented by vaccination but protection rates of the available vaccines are far from satisfying. Although well-working vaccines have been developed for other infectious diseases such as smallpox or polio without understanding the underlying immunology, this has been proven to be difficult for typhoid fever. There are three vaccines licensed of which two are commercially available. Currently, a life-attenuated vaccine strain (Ty21a) that lacks the virulent Vi antigen and a parenteral Vi polysaccharide vaccine are used [ 5 , 6 ]. A recent Cochrane meta-analysis identified 17 randomized trials about typhoid vaccines and showed both vaccines to be equally effective. Three doses of the oral Ty21a vaccine provided a protection rate of 34 to 58%. While the parenteral vaccine showed a cumulative efficacy of 30 to 70% for two years [ 7 ]. The Vi vaccine is recommended by the WHO, although controversies on its safety and efficacy in younger children exist [ 8 , 9 ]. Of note, these efficacy rates cannot be directly used for travellers as the data was obtained from local populations. Only an early trial with 20 participants calculated a 95% protection rate in travellers [ 10 ].
In endemic countries, the diagnosis of typhoid fever is often based upon the clinical presentation [ 5 ]. The diagnostic gold standard remains the bacterial culture especially from the bone marrow area [ 4 ]. The classical serologic test - still used in many parts of the world - was first described in 1896 by Felix Widal (the Widal test). This agglutination assay uses the H (flagellar), O (somatic) - and in newer versions also the Vi capsular antigens. Its easy and cheap usage is counterbalanced by unsatisfying sensitivity and specificity [ 11 , 12 ]. In general, a four-time rise within seven to ten days in the agglutinin titre is considered to be a positive test result [ 13 ]. Interestingly, S. typhi and the attenuated Ty21 vaccine strain both express H and O antigens [ 5 ]. In contrast, vaccination with the attenuated vaccine strain resulted in a positive agglutinin test in less than 60% of the participants, only [ 13 ]. Moreover, other infectious diseases such as dengue fever or malaria can cause false positive results and positive test results might reflect previous infection. In a review about diagnostic tests for typhoid fever, Olopoenia et al. advised that the Widal test in endemic areas should only be considered positive when a fourfold increase in titres within two to three weeks is observed and argues against the usefulness of a single test [ 14 ].
As stated by the WHO 40 years ago, '… host defence mechanisms in human typhoid infection are poorly understood and the nature of protective immunity is largely unknown…’ [ 15 ]. To date, no correlation of antibody titres to protection against infection or disease could be established and the role of antibodies is unclear [ 16 ]. Sarasombath et al. investigated the antibody response after typhoid infection and measured anti-O and anti-H agglutinins using the Widal test. All patients developed anti-O agglutinin between week eight and week ten of the disease. But there were three patients with no seroconversion by week four of infection. Moreover anti-H agglutinin was found in all but two patients at week four of the disease [ 17 ]. The authors proposed that the presence of O- and H-antigens do not provide full protection and postulated a crucial role for a cellular-mediated immune response [ 17 ]. In another early work by Dham et al. immune responses after parenteral vaccination and infection were investigated. Although the authors did not especially report the number of patients without seroconversion, apparently, there was at least one patient without the expected rise in H- and O-antibody titres [ 18 ]. Thus, some patients, including our case, fail to develop antibody titres. Recently, Lindow et al. reported a complex action of typhoid-specific antibodies after vaccination using a new single-dose oral vaccine (M01ZH09) that is under investigation but not yet licensed [ 16 ]. In their model, antibodies generated against the vaccine strain resulted in increased uptake and killing of S. typhi in macrophage cells and increased complement-mediated killing of the bacteria [ 16 ].
Interestingly, S. typhi and the attenuated Ty21 vaccine strain both express H- and O-antigens [ 5 ]. In the case presented, our patient did not develop an antibody response against these antigens although being exposed twice - once upon vaccination and once upon infection.
Moreover, other infectious diseases such as dengue fever or malaria can cause false positive results and positive test results might reflect previous infection. In their review about diagnostic tests for typhoid fever, Olopoenia et al. advised against the routine usage of the Widal test [ 14 ].
We report a case of a young otherwise healthy traveller who had Salmonella bacteraemia and clinical signs of systemic infection. She presented with typical clinical signs of typhoid fever such as high fever, constipation and a dry cough. Initial laboratory diagnostics showed eosinophilia and a relatively low leucocyte count (no neutropenia) as a potential diagnostic marker for typhoid fever. Although being vaccinated, she did not develop a protective immunity nor antibody titres against H- and O- antigens after infection. After all, our case underscores the need for a better understanding of the immune responses that occur in typhoid fever. Recent advances and the development of a new animal model of typhoid fever are promising. Using a chimeric rag-2 deficient mouse with humanized hematopoietic stem and progenitor cells [ 19 ] may help to shed light on the so far insufficiently understood immune response and development of immunity to typhoid fever and finally lead to more reliable tests and better vaccines.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Paz A, Cohen E, Odeh M, Potasman I: Typhoid fever in travellers: time for reassessment. Eur J Intern Med. 2007, 18: 150-151. 10.1016/j.ejim.2006.09.016.
Article PubMed Google Scholar
Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E, GeoSentinel Surveillance Network: Fever in returned travellers: results from the GeoSentinel surveillance network. Clin Infect Dis. 2007, 44: 1560-1568. 10.1086/518173.
Robert Koch Institute: Current statistics of mandatory reportings of infectious diseases in Germany, 2012. Epid Bull. 2013, 3: 30-
Google Scholar
Crump JA, Mintz ED: Global trends in typhoid and paratyphoid fever. Clin Infect Dis. 2010, 50: 241-246. 10.1086/649541.
Article PubMed PubMed Central Google Scholar
Connor BA, Schwartz E: Typhoid and paratyphoid fever in travellers. Lancet Inf Dis. 2005, 5: 623-628. 10.1016/S1473-3099(05)70239-5.
Article Google Scholar
House D, Ho VA, Diep TS, Chinh NT, Bay PV, Vinh H, Duc M, Parry CM, Dougan G, White NJ, Farrar JJ, Hien TT, Wain J: Antibodies to the Vi capsule of Salmonella Typhi in the serum of typhoid patients and healthy control subjects from a typhoid endemic region. J Infect Dev Ctries. 2008, 2: 308-312.
PubMed Google Scholar
Fraser A, Goldberg E, Acosta CJ, Paul M, Leibovici L: Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2007, CD001261-
Podda A, Saul AJ, Arora R, Bhutta Z, Sinha A, Gaind R, Singhal T, Saha S, Brooks A, Martin LB, Amdekar Y, Chitkara AJ, Shieh M, Kapur AN, Chugh TD: Conjugate vaccines for enteric fever: proceedings of a meeting organized in New Delhi, India in 2009. J Infect Dev Ctries. 2010, 4: 404-411.
Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, Dutta S, Donner A, Kanungo S, Park JK, Puri MK, Kim DR, Dutta D, Bhaduri B, Acosta CJ, Clemens JD: A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Eng J Med. 2009, 361: 335-344. 10.1056/NEJMoa0807521.
Article CAS Google Scholar
Schwartz E, Shlim DR, Eaton M, Jenks N, Houston R: The effect of oral and parenteral typhoid vaccination on the rate of infection with Salmonella typhi and Salmonella paratyphi A among foreigners in Nepal. Arch Intern Med. 1990, 150: 349-351. 10.1001/archinte.1990.00390140079017.
Article CAS PubMed Google Scholar
Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ: Typhoid fever. N Engl J Med. 2002, 347: 1770-1782. 10.1056/NEJMra020201.
Somily AM, Adam MH, Gad El Rab MO, Morshed MG, Shakoor Z: Detection of Salmonella typhi agglutinins in sera of patients with other febrile illnesses and healthy individuals. Ann Afr Med. 2011, 10: 41-44. 10.4103/1596-3519.76584.
Sarasombath S, Banchuin N, Sukosol T, Vanadurongwan S, Rungpitarangsi B, Dumavibhat B: Systemic and intestinal immunities after different typhoid vaccinations. Asian Pac J Allergy Immunol. 1987, 5: 53-61.
CAS PubMed Google Scholar
Olopoenia LA, King AL: Widal agglutination test – 100 years later: still plagued by controversy. Postgrad Med J. 2000, 76: 80-84. 10.1136/pmj.76.892.80.
Article CAS PubMed PubMed Central Google Scholar
Oral enteric bacterial vaccines. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1972, 500: 1-34.
Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD: Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect Immun. 2011, 79: 3188-3194. 10.1128/IAI.05081-11.
Sarasombath S, Banchuin N, Sukosol T, Rungpitarangsi B, Manasatit S: Systemic and intestinal immunities after natural typhoid infection. J Clin Microbol. 1987, 25: 1088-1093.
CAS Google Scholar
Dham SK, Thompson RA: Studies of cellular and humoral immunity in typhoid and TAB vaccinated subjects. Clin Exp Immunol. 1982, 48: 389-395.
CAS PubMed PubMed Central Google Scholar
Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galán JE: A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010, 8: 369-376. 10.1016/j.chom.2010.09.003.
Download references
Author information
Authors and affiliations.
Department of Internal Medicine, Neurology and Dermatology, Medical Intensive Care Unit, University Hospital Leipzig, Liebigstrasse 20, 04103, Leipzig, Germany
Martin Grimm
Department of Internal Medicine, Neurology and Dermatology, Clinic of Gastroenterology and Rheumatology, University Hospital Leipzig, Liebigstrasse 20, 04103, Leipzig, Germany
Martin Grimm, Christoph Lübbert, Joachim Mössner & Sebastian Weis
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Martin Grimm .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
MG and SW collected the data and drafted the manuscript. CL contributed to the clinical and scientific content of the work. JM critically revised the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Grimm, M., Lübbert, C., Mössner, J. et al. A young traveller presenting with typhoid fever after oral vaccination: a case report. J Med Case Reports 7 , 237 (2013). https://doi.org/10.1186/1752-1947-7-237
Download citation
Received : 19 April 2013
Accepted : 20 August 2013
Published : 07 October 2013
DOI : https://doi.org/10.1186/1752-1947-7-237
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vaccine Strain
- Typhoid Fever
- Dengue Fever
- Positive Test Result
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Typhoid fever as a cause of opportunistic infection: case report
Claudia colomba, laura saporito, laura infurnari, salvatore tumminia, lucina titone.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2005 Jul 18; Accepted 2006 Feb 27; Collection date 2006.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Typhoid fever is a systemic infection caused by the bacterium Salmonella enterica subspecies enterica serotype typhi , which is acquired by ingestion of contaminated food and water. Each year the disease affects at least 16 million persons world-wide, most of whom reside in the developing countries of Southeast Asia and Africa. In Italy the disease is uncommon with a greater number of cases in Southern regions than in Northern ones.
Case presentation
We report on a 57-year-old Sri-Lankan male affected by typhoid fever, the onset of which was accompanied by oropharyngeal candidiasis. This clinical sign was due to a transient cell-mediated immunity depression (CD4+ cell count was 130 cells/mm 3 ) probably caused by Salmonella typhi infection. Human immunodeficiency virus infection was ruled out. Diagnosis of typhoid fever was made by the isolation of Salmonella typhi from two consecutive blood cultures. The patient recovered after a ten days therapy with ciprofloxacin and his CD4+ cell count improved gradually until normalization within 3 weeks.
Our patient is the first reported case of typhoid fever associated with oropharyngeal candidiasis. This finding suggests a close correlation between Salmonella typhi infection and transitory immunodepression.
Typhoid fever is a systemic infection caused by the bacterium Salmonella enterica subspecies enterica serotype Typhi , which is acquired by ingestion of contaminated food and water. Each year the disease affects at least 16 million persons world-wide, most of whom reside in the developing countries of Southeast Asia and Africa [ 1 ]. Typhoid fever is uncommon in industrialised regions such as the USA, Canada, Europe, Australia and Japan and new cases of the disease in these countries are related to travel to developing countries [ 2 , 3 ]. Based on such data, the public health authorities in most industrialised countries recommend vaccination against typhoid fever for travellers to the developing world, where sanitary conditions are poor [ 4 ]. Italy is a low endemicity country for typhoid fever and a greater number of cases occur in Southern regions than in Northern ones. Sicily is the second region of our country for incidence, with a mean of 100 annual cases in the last five years.
Although typhoid fever is classically described as an acute illness with fever and abdominal tenderness, the symptoms are non specific and may be insidious in onset [ 5 ]. The diagnosis of enteric fever should be seriously considered in the evaluation of travellers who return from tropical and subtropical areas with fever. Mortality rates associated with typhoid fever vary from region to region, with the highest reported from Indonesia, Nigeria, and India [ 6 ].
We report on an imported case of typhoid fever, the onset of which was accompanied by oropharyngeal candidiasis.
In November 2004, a 57-year-old Sri-Lankan male turned up at the emergency department of our hospital. The patient had been living in Italy for 14 years, when he went back home to Sri-Lanka. He was there for 2 months, before returning to Italy where, few days later, he began suffering from fever, malaise, headache and non productive cough. He turned up at the emergency department of our hospital after 10 days from the beginning of symptoms.
On the basis of anamnestic data no history of drinking, smoking and illicit drug abuse was reported. Moreover, in his medical history there was no evidence of serious illness, such as diabetes mellitus or human immunodeficiency virus (HIV) infection. The patient had not been using proton pump inhibitors of stomach acid tablets, neither oral glycocorticosteroids or inhalation corticosteroids. Moreover, he did not need to take any antibiotics during his stay in Sri-Lanka.
The patient had a blood pressure of 180/100 mm Hg, a heart rate of 120 beats/min, a respiratory rate of 16 breaths/min, and a temperature of 38.9°C. On chest examination bilateral scattered rhonchi and mild rales were audible at the base of both the man's lungs. A chest radiograph revealed accentuation of the pulmonary reticulum, more marked on the right, but no clear signs of consolidation. The rest of the examination was notable for a minimally distended abdomen which was diffusely tender. Laboratory examination revealed a white blood cells (WBC) count of 6,400 cells/mm 3 (normal range 4,000–10,000 cells/ mm 3 ), a haemoglobin level of 15 g/dl (12–17 g/dl), a platelet count of 189,000 cells/mm 3 (150,000 – 450,000 cells/mm 3 ), an aspartate amino-transferase level of 71 U/L, an alanine aminotransferase level of 98 U/L; blood glucose level, serum electrolyte concentrations and renal function tests were within normal limits. A presumptive diagnosis of bronchitis was made, and the patient was transferred to our infectious disease department.
At the time of admission, the patient was persistently febrile (39°C). On physical examination we noted dry skin and oral candidiasis characterized by creamy white, curdlike patches on the tongue; this clinical picture was so typical that it did not need a culture to confirm the diagnosis of oral candidiasis. His abdomen was diffusely tender to palpation and without recognizable hepatosplenomegaly. Cultures of blood and respiratory specimens were performed. Thick and thin blood smears for malaria were obtained.
Treatment with topical nystatin and empirical therapy with ciprofloxacin (500 mg iv every 12 hours) were started. Because of the oro pharyngeal candidiasis, lymphocyte count was taken, the absolute number of lymphocytes was 562 cells/ mm 3 . The CD4+ cell count was 130 cells/mm 3 while the CD8+ was 240; as a second step an HIV test (ELISA) was performed and was negative. To rule out a very recent infection a qualitative HIV PCR was performed and HIV RNA was undetectable. On the 3rd day of hospitalisation, a Salmonella species identified as S. typhi was isolated from the two consecutive blood cultures obtained on the day of admission. Isolates were susceptible to ampicillin, cotrimoxazole, chloramphenicol, cefotaxime, ceftriaxone, and ciprofloxacin with the standard disk diffusion susceptibility testing method. The patient continued ciprofloxacin for a total of 10 days; he became afebrile after 3 days of therapy and his oral candidiasis improved slowly with nistatin. The patient recovered and was discharged from the hospital on the 10th day. At that time, his CD4+ cell count was 622 cells/mm 3 .
On the 20th day after discharge and in the 3rd month of follow-up, the patient was symptom free and his CD4+ cell count was raised to 1,080 and 1,200 cells/mm 3 respectively.
In Italy, typhoid fever has become an uncommon disease occurring mainly as a sporadic disease in patients of any age and gender, and at any time of the year. The prevalence rate of typhoid fever has markedly decreased, from nearly 10,000 documented cases annually in the 1970s to a few hundred cases annually in the 2000s [ 7 , 8 ]. This data suggest that Italy is still a low endemicity country for typhoid fever, because of the presence of a moderate number of autochthonous cases mainly reported in the Southern regions of our country [ 9 ]. Nevertheless, S. typhi infection is one of the major causes of fever in patients admitted in hospital after returning from the tropics. Together with hepatitis A it represents the cause of a significant number of hospitalizations that pretravel vaccination strategies could have prevented [ 10 ].
Recently many strains of multidrug resistant (MDR) S. typhi have been identified worldwide [ 11 - 14 ]; in Italy an epidemiologic study conducted between 1980 and 1996 showed that their spread was until now quite low [ 15 ]. However an accurate and continuous surveillance is necessary in order to quickly identify MDR S. typhi strains and prevent their spread.
We report on an imported case of typhoid fever as previously described in other developed countries [ 16 ].
Patients with typhoid fever usually have a history of prolonged fever, headache and abdominal discomfort. There are no distinctive clinical features, and definitive diagnosis requires isolation of S . typhi from blood, bone marrow, stool, or urine cultures [ 5 ]. Our patient had fever with upper airway symptoms and showed typical lesions observed in oral candidiasis.
To date, oropharyngeal candidiasis is an opportunistic infection that generally develops in patients who have been using inhaled steroids over a long period of time, and those with cancer and AIDS [ 17 , 18 ]. Because our patient did not belong to any of the three categories of patients reported above, we performed an HIV test to rule out any recent HIV infection; the result was negative [ 19 ]. We noticed that the CD4+ cell count was low in the acute phase of disease and improved gradually until normalization within 3 weeks. These findings support the hypothesis that S. typhi could lead to a progressive reduction of CD4+ T helper cell population as already demonstrated by a previous study performed on mice infected with S. typhimurium [ 20 ].
In conclusion, to our knowledge our patient is the first reported case of typhoid fever the onset of which was accompanied by oropharyngeal candidiasis, suggesting a close correlation between S. typhi infection and transitory immunodepression.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
All authors contributed equally to this work

Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/1471-2334/6/38/prepub
Acknowledgments
Acknowledgements.
Written consent was obtained from the patient for publication of this case report.
Contributor Information
Claudia Colomba, Email: [email protected].
Laura Saporito, Email: [email protected].
Laura Infurnari, Email: [email protected].
Salvatore Tumminia, Email: [email protected].
Lucina Titone, Email: [email protected].
- Centers for Diseases Control and Prevention Traveler's health Preventing typhoid fever: a guide for travellers Atlanta. 2003. http://www.cdc.gov/travel/diseases/typhoid.htm
- Papadimitropoulos V, Vergidis PI, Bliziotis I, Falagas ME. Vaccination against typhoid fever in travellers: a cost-effectiveness approach. Clin Microbiol Infect. 2004;10:681–683. doi: 10.1111/j.1469-0691.2004.00901.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Taylor DN, Pollard RA, Blake PA. Typhoid in the United States and the risk to the international travel. J Infect Dis. 1983;148:599–602. doi: 10.1093/infdis/148.3.599. [ DOI ] [ PubMed ] [ Google Scholar ]
- Centers for Disease Control and Prevention Health information for international travel 1996–1997 Atlanta. 1996.
- Parry CM, Hien TT, Dougan G, White NJ, Farran JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1780. doi: 10.1056/NEJMra020201. [ DOI ] [ PubMed ] [ Google Scholar ]
- Miller SI, Hohmann EL, Pegues DA. Salmonella (including Salmonella Typhi) In: Mandell GL, Bennet JR, Dolin R, editor. Principles and practice of infectious diseases. 4. Vol. 2. New York: Livingstone; 1994. pp. 2013–2033. [ Google Scholar ]
- Bollettino Epidemiologico Nazionale. Ministero della Salute http://www.ministerosalute.it/promozione/malattie/bollettino.jsp
- Scuderi G. A review of the Salmonellosis surveillance systems in Italy: evolution during the course of time within the international framework. Eur J Epidemiol. 2000;16:861–8. doi: 10.1023/A:1007678901475. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rizzo G, De Vito D. Typhoid fever and environmental contamination in Apulia Region, Italy. Ann Ig. 2003;15:487–92. [ PubMed ] [ Google Scholar ]
- Antinori S, Galimberti L, Gianelli E, Calattini S, Piazza M, Morelli P, Moroni M, Galli M, Corbellino M. Prospective observational study of fever in hospitalized returning travelers and migrants from tropical areas, 1997–2001. J Travel Med. 2004;11:135–42. doi: 10.2310/7060.2004.18557. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nkemngu NJ, Asonganyi EDN, Njunda AL. Treatment failure in a typhoid patient infected with nalidixic acid resistant S. enterica serovar Typhi with reduced susceptibility to Ciprofloxacin: a case report from Cameroon. BMC Infect Dis. 2005;5:49. doi: 10.1186/1471-2334-5-49. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Akinyemi KO, Smith SI, Oyefolu AO, Coker AO. Multidrug resistance in Salmonella enterica serovar typhi isolated from patients with typhoid fever complications in Lagos, Nigeria. Public Health. 2005;119:321–7. doi: 10.1016/j.puhe.2004.04.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- Shakespeare WA, Davie D, Tonnerre C, Rubin MA, Strong M, Petti CA. Nalidixic acid-resistant Salmonella enterica serotype Typhi presenting as a primary psoas abscess: case report and review of the literature. J Clin Microbiol. 2005;43:996–8. doi: 10.1128/JCM.43.2.996-998.2005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huang DB, DuPont HL. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis. 2005;5:341–8. doi: 10.1016/S1473-3099(05)70138-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Scuderi G, Fantasia M, Niglio T. The antibiotic resistance patterns of Salmonella Typhi isolates in Italy, 1980–96. The Italian SALM-NET Working Group. Salmonella Network. Epidemiol Infect. 2000;124:17–23. doi: 10.1017/S0950268899003301. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Su CP, Chen YC, Chang SC. Changing characteristics of typhoid fever in Taiwan. J Microbiol Immunol Infect. 2004;37:109–114. [ PubMed ] [ Google Scholar ]
- Simon MR, Houser WL, Smith KA, Long PM. Esophageal candidiasis as a complication of inhaled corticosteroids. Ann Allergy Asthma Immunol. 1997;79:333–338. doi: 10.1016/S1081-1206(10)63024-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Maenza JR, Merz WG, Romagnoli MJ, Keruly JC, Moore RD, Gallant JE. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin Infect Dis. 1997;24:28–34. doi: 10.1093/clinids/24.1.28. [ DOI ] [ PubMed ] [ Google Scholar ]
- Syrjanen S, Valle SL, Antonen J, Suni J, Saxinger C, Krohn K, Ranki A. Oral candidal infection as a sign of HIV infection in homosexual men. Oral surg. 1988;55:36–40. doi: 10.1016/0030-4220(88)90188-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gupta S. Priming of T-cell responses in mice by porins of Salmonella typhimurium. Scandinavian J Immunol. 1988;48:136–143. doi: 10.1046/j.1365-3083.1998.00326.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (197.6 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Presentations
Typhoid Fever is most common on the East Coast of the United States and almost all of Europe.
The side effects to typhoid fever are deadly and not so deadly . The infected person may think he/she has contracted the flu or severe cold. An instant headache occurs during this process.
As I said before there is a dangerous part to this. The dangerous part of it is that it slows down your heart rate so at any given moment you can collapse. another dangerous side effect is that your stomach starts to bleed and your intestines could blow up/explode inside of you. the only part you could physically see on your body when you have typhoid fever is rosy spots on your arms and legs.
Now all those symptoms aren't going to stay with you for ever. Theres a cure!
In 1851 a scientist by the name of William Jenner created a vaccine that would cure typhoid fever. William did not give this vaccine a name.
Vaccination isnt the only way you could cure it. you could also cure typhoid fever by tremenous feeding.
If you want to prevent typhoid fever make sure you always have proper sanitation of food water and other resources.
By this point you may be wondering. What is typhoid fever! And how did it even get here!
Well heres the history behind it.
Around 430-424 BC, a devestating plague which some believe to have been "typhoid fever, killed one third of the population of Athens, including their lead Pericles. The balance of power shifted from Athens to Sparta, ending the Golden Age of Pericles that ahd marked Athenian dominance in the ancient world.
and that, is typhoid fever
Typhoid fever.
By alaniguzman
alaniguzman
More from alaniguzman.


- Epidemiology.
Estimated incidence of typhoid and paratyphoid fevers by country per 100,000 population, 2015.
Estimated global mortality from typhoid and paratyphoid fever by country per million, 2015.
- View raw image
- Download Powerpoint Slide
Buckle GC , Walker CL , Black RE , 2012 . Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010 . J Glob Health 2 : 010401 .
- Search Google Scholar
- Export Citation
European Centre for Disease Prevention and Control . Facts About Typhoid Fever and Paratyphoid Fever . Available at: https://ecdc.europa.eu/en/typhoid-and-paratyphoid-fever/facts . Accessed May 10, 2017.
Global Burden of Disease , 2018 . Global Burden of Disease Results Tool . GBD Results Tool | GHDx. Available at: http://ghdx.healthdata.org/gbd-results-tool . Accessed May 10, 2017.
Mogasale V , Maskery B , Ochiai RL , Lee JS , Mogasale VV , Ramani E , Kim YE , Park JK , Wierzba TF , 2014 . Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment . Lancet Glob Health 2 : e570 – e580 .
Crump JA , Luby SP , Mintz ED , 2004 . The global burden of typhoid fever . Bull World Health Organ 82 : 346 – 353 .
Ministry of Health Thailand (MoH), Bureau of Epidemiology, Department of Disease Control , 2018 . Typhoid Surveillance . Available at: http://www.boe.moph.go.th/boedb/surdata/disease.php?ds=08 . Accessed March 1, 2018.
Ministry of Health Thailand (MoH), Bureau of Epidemiology, Department of Disease Control , 2018 . Paratyphoid Surveillance . Available at: http://www.boe.moph.go.th/boedb/surdata/disease.php?ds=09 . Accessed March 1, 2018.
One World—Nations Online , 2009 . Political Map of Thailand . Available at: http://www.nationsonline.org/oneworld/map/thailand-region-map.htm . Accessed March 1, 2018.
Date KA , Newton AE , Medalla F , Blackstock A , Richardson L , McCullough A , Mintz ED , Mahon BE , 2016 . Changing patterns in enteric fever incidence and increasing antibiotic resistance of enteric fever isolates in the United States, 2008–2012 . Clin Infect Dis 63 : 322 – 329 .
Center for Disease Control , 2014 . Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet Surveillance Report for 2014 (Final Report) . Atlanta, Georgia: US Department of Health and Human Services, CDC.
Ochiai RL et al. Domi Typhoid Study Group , 2008 . A study of typhoid fever in five Asian countries: disease burden and implications for controls . Bull World Health Organ 86 : 260 – 268 .
Lin FY , Vo AH , Phan VB , Nguyen TT , Bryla D , Tran CT , Ha BK , Dang DT , Robbins JB , 2000 . The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam . Am J Trop Med Hyg 62 : 644 – 648 .
Naheed A , Ram PK , Brooks WA , Hossain MA , Parsons MB , Talukder KA , Mintz E , Luby S , Breiman RF , 2010 . Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh . Int J Infect Dis 14 ( Suppl 3 ): e93 – e99 .
Als D , Radhakrishnan A , Arora P , Gaffey MF , Campisi S , Velummailum R , Zareef F , Bhutta ZA , 2018 . Global trends in typhoidal salmonellosis: a systematic review . Am J Trop Med Hyg 99 ( Suppl 3 ): 10 – 19 .
Breiman RF et al. 2012 . Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa . PLoS One 7 : e29119 .
Marks F et al. 2017 . Incidence of invasive Salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study . Lancet Glob Health 5 : e310 – e323 .
Wain J , Hendriksen RS , Mikoleit ML , Keddy KH , Ochiai RL , 2015 . Typhoid fever . Lancet 385 : 1136 – 1145 .
GDB 2015 Mortality and Causes of Death Collaborators , 2016 . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 . Lancet 388 : 1459 – 1544 .
Bhutta ZA , 1996 . Impact of age and drug resistance on mortality in typhoid fever . Arch Dis Child 75 : 214 – 217 .
Siddiqui FJ , Haider SR , Bhutta ZA , 2008 . Risk factors for typhoid fever in children in squatter settlements of Karachi: a nested case-control study . J Infect Public Health 1 : 113 – 120 .
Ochiai RL et al. 2007 . The use of typhoid vaccines in Asia: the DOMI experience . Clin Infect Dis 45 ( Suppl 1 ): S34 – S38 .
Bodhidatt L , Taylor DN , Thisyakorn U , Echeverria P , 1987 . Control of typhoid fever in Bangkok, Thailand, by annual immunization of schoolchildren with parenteral typhoid vaccine . Rev Infect Dis 9 : 841 – 845 .
Parry CM , Wijedoru L , Arjyal A , Baker S , 2011 . The utility of diagnostic tests for enteric fever in endemic locations . Expert Rev Anti Infect Ther 9 : 711 – 725 .
Al-Emran HM et al. 2016 . Validation and identification of invasive Salmonella serotypes in sub-saharan Africa by multiplex polymerase chain reaction . Clin Infect Dis 62 ( Suppl 1 ): S80 – S82 .
Mogasale V , Ramani E , Mogasale VV , Park J , 2016 . What proportion of Salmonella typhi cases are detected by blood culture? A systematic literature review . Ann Clin Microbiol Antimicrob 15 : 32 .
Lee A , Mirrett S , Reller LB , Weinstein MP , 2011 . Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol 45 : 3546 – 3548 .
Wain J , Hosoglu S , 2008 . The laboratory diagnosis of enteric fever . J Infect Dev Ctries 2 : 421 – 425 .
World Health Organization (WHO) , 2003 . Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever . Communicable Disease Surveillance and Response Vaccines and Biologicals. Available at: http://www.who.int/rpc/TFGuideWHO.pdf . Accessed June 19, 2018.
Wain JDT , Bay PV , Walsh AL , Vinh H , Duong NM , Ho VA , Hien TT , Farrar J , White NJPC , Day NP , 2008 . Specimens and culture media for the laboratory diagnosis of typhoid fever . J Infect Dev Ctries 2 : 469 – 474 .
Harish BN , Menezes GA , 2011 . Antimicrobial resistance in typhoidal salmonellae . Indian J Med Microbiol 29 : 223 – 229 .
World Health Organization (WHO) , 2011 . Guidelines for the Management of Typhoid Fever . Available at: http://apps.who.int/medicinedocs/documents/s20994en/s20994en.pdf . Accessed May 10, 2017.
Bhan MK , Bahl R , Bhatnagar S , 2005 . Typhoid and paratyphoid fever . Lancet 366 : 749 – 762 .
Capoor MR , Nair D , Posti J , Singhal S , Deb M , Aggarwal P , Pillai P , 2009 . Minimum inhibitory concentration of carbapenems and tigecycline against Salmonella spp . J Med Microbiol 58 : 337 – 341 .
Shrestha KL , Pant ND , Bhandari R , Khatri S , Shrestha B , Lekhak B , 2016 . Re-emergence of the susceptibility of the Salmonella spp. isolated from blood samples to conventional first line antibiotics . Antimicrob Resist Infect Control 5 : 22 .
World Health Organization (WHO) , 2017 . Typhoid Fever 2016 . Available at: http://www.who.int/ith/vaccines/typhoidfever/en/ . Accessed May 10, 2017.
Jin C et al. 2017 . Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial . Lancet 390 : 2472 – 2480 .
Levine MM , Ferreccio C , Cryz S , Ortiz E , 1990 . Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial . Lancet 336 : 891 – 894 .
Acharya IL et al. 1987 . Prevention of typhoid fever in Nepal with the VI capsular polysaccharide of Salmonella typhi . N Engl J Med 317 : 1101 – 1104 .
Yang HH et al. 2001 . Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China . Bull World Health Organ 79 : 625 – 631 .
Related Content
Tacaribe virus, a new agent isolated from artibeus bats and mosquitoes in trinidad, west indies, increase in size of entamoeba hartmanni trophozoites cultured on an enriched medium, two nosological forms of cutaneous leishmaniasis, experimental intrahepatic portal embolism induced by adult schistosoma mansoni, ethanol extracts of various helminths in a complement fixation test for eosinophilic lung (tropical eosinophilia).

- Previous Article
- Next Article
Introductory Article on Global Burden and Epidemiology of Typhoid Fever
- Download PDF
This article is the introduction to a 12-paper supplement on global trends in typhoid fever. The Tackling Typhoid (T2) project was initiated in 2015 to synthesize the existing body of literature on typhoidal salmonellae and study national and regional typhoid fever trends. In addition to a global systematic review, eight case studies were undertaken to examine typhoid and paratyphoid fever trends in endemic countries alongside changes in relevant contextual factors. Incidence variations exist both within and between regions with large subnational differences as well, suggesting that public health changes impacting typhoid and paratyphoid fevers in one setting may not have similar impacts in another. This supplement also brings to light the lack of national typhoid fever surveillance systems, inconsistencies in diagnostics, and the lack of typhoid fever associated morbidity and mortality data in many countries, making it difficult to accurately quantify and track burden of disease. To better understand typhoid fever there is a need for more high-quality data from resource-poor settings. The implementation of typhoid surveillance systems alongside the transition to blood-culture confirmation of cases, where possible, would aid in the improvement of data quality in low-income settings. The following supplement includes the results of our global systematic review, eight-country case study articles, a qualitative article informed by semistructured interviews, and a conclusion article on potential ways forward for typhoid control.
INTRODUCTION
This article introduces a collection of studies undertaken within the “Tackling Typhoid” (T2) project, which aimed to consolidate the current body of literature on national and regional typhoid fever trends in incidence, mortality, and severe complications. The T2 project included a systematic review of the literature and eight-country case studies in Chile, Nigeria, South Africa, Pakistan, India, Bangladesh, Thailand, and Vietnam. Case studies examined typhoid and paratyphoid fever incidence trends in conjunction with changes in relevant contextual factors, such as water treatment and distribution, sanitation infrastructure, female literacy, poverty rates, and diarrheal mortality, and included in-depth interviews with local public health experts to identify local interventions and control measures that were implemented to reduce the transmission of typhoid fever directly or indirectly by targeting other infectious disease. The studies in this supplement collectively characterize global and regional trends in typhoid and paratyphoid fever, and the findings can inform recommendations on interventions and policies that can best help curb the continued spread of this disease.
Typhoid and paratyphoid fever are enteric infections caused by the bacteria Salmonella enterica serovar Typhi ( S. Typhi) and Paratyphi A, B, and C ( S. Paratyphi A, B, and C), respectively, collectively referred to as typhoidal Salmonella , and the causes of enteric fever. 1 Humans are the only reservoir for Salmonella Typhi with disease transmission occurring via the fecal–oral route, usually through the consumption of food or water contaminated by human feces. 2 An estimated 17 million cases of typhoid and paratyphoid fever illnesses occurred globally in 2015, 3 mostly in South Asia, Southeast Asia, and sub-Saharan Africa, with both the largest burden and incidence occurring in South Asia ( Figure 1 ). 3 , 4 Left untreated, both typhoid and paratyphoid fever may be fatal 1 with 178,000 deaths estimated worldwide in 2015. 3
Citation: The American Journal of Tropical Medicine and Hygiene 99, 3_Suppl; 10.4269/ajtmh.18-0032
- Download Figure
- Download figure as PowerPoint slide
Although considerable literature exists on typhoid fever incidence, most endemic countries do not have well-established population-based national surveillance systems for typhoid fever. In addition, some countries that use passive surveillance use clinical diagnoses with limited ability to confirm typhoid fever cases by blood culture. Most data are, therefore, collected from hospital-based studies, leaving substantial knowledge gaps in certain geographies, especially where health-care usage is low. A review of global burden 5 showed that from 1954 to 2000 only 13 countries had population-based surveillance data for typhoid fever, only two of which were in Africa—Egypt and South Africa. At the time, both these countries had surveillance data from the control arms of vaccination trials only, 5 although considerably more data have become available since. Data from Ministry of Health surveillance reports in Thailand highlight a shift from S. Typhi as the primary typhoidal Salmonella bacteria isolated to S. Paratyphi. 6 Thailand is broken down into seven regions, of which four are showing this transition between 2004 and 2014. 6 – 8 Within the Bangkok and Vicinities region, two provinces (Bangkok and Samut Prakan) of the six that comprise the region show S. Typhi incidence decreasing as S. Paratyphi increases. 6 , 7 This shift is also observed in three provinces (Ratchaburi, Kanchanaburi, and Phetchaburi) from the western region of Thailand. 6 , 7 Although improvements in water, sanitation infrastructure, and public health measures have led to the virtual disappearance of typhoid fever transmission within the developed world, residual cases largely occur in travelers returning from countries where typhoid fever remains endemic. 9 Knowledge of local disease burden, risk factors for acquisition, transmission characteristics, and implemented control measures are essential in developing strategies for prioritized and optimally targeted typhoid and paratyphoid fever control, and elimination.
Typhoid fever incidence has decreased to very low levels in developed countries such as the United States and Canada. 9 In 1990, surveillance from the United States recorded incidence at 0.22 cases per 100,000 persons per year. Incidence has since decreased even further and remained consistently below 0.16 cases per 100,000 persons per year as of 1995. 10 A study assessed 1,872 cases of typhoid fever from 2008 to 2012 of which 86% were associated with foreign travel. The small number of cases still being acquired domestically demonstrates that typhoid fever remains a constant, albeit minor problem within the United States. 9
Although most cases of typhoid fever occur in Asia and Africa, considerable regional differences exist, both within and between countries. Data from the Diseases of the Most Impoverished (DOMI) population-based surveillance study, led by the International Vaccine Institute (Seoul, South Korea), estimated the overall incidence of 493.5 cases per 100,000 person-years in children aged 5–15 years in an urban slum in Kolkata, India (2003–2004). 11 A similar population-based study conducted in the Dong Thap Province in the south of Vietnam from December 1995 to December 1996 estimated typhoid fever incidence at 198 per 100,000 person-years. 12 A comparable incidence was observed in Dhaka between January 2003 and January 2004 where typhoid fever incidence was estimated to be 200 cases per 100,000 person-years. 13 Most previous typhoid fever estimates from sub-Saharan African countries were from hospital-based studies, 14 although earlier population-based studies from Kenya did suggest high rates in an urban slum of 247 cases per 100,000 person-years observed between 2007 and 2009. This study also showed 15-fold higher incidence in urban children compared with their rural counterparts. 15 A recent publication describes population-based studies of typhoid fever and invasive nontyphoidal Salmonella disease in 12 sites in 10 countries across sub-Saharan Africa. 16 Incidence estimates ranged from 0 to 383 cases per 100,000 person-years across the 12 sites. Although poor infrastructure and unstable governments make the establishment of robust disease surveillance systems difficult in resource-constrained settings, 17 these new data from sub-Saharan Africa will help in forming more accurate future estimations of the global burden.
Age-specific typhoid incidence.
Typhoid fever incidence varies by age. In endemic countries, the highest incidence is in younger children, whereas incidence is similar in all age groups in low-burden settings. A study from 2004 used data from published studies to extrapolate incidence rates by age group and reported the highest incidence in children under the age of 5 years in high incidence settings. 5 Modeled estimates from the 2015 Global Burden of Disease study (GBD 2015) showed typhoid fever incidence rates decreasing as age increased. 3 Furthermore, results from the DOMI study conducted in five endemic countries demonstrate substantial heterogeneity in typhoid fever incidence across age groups. The heterogeneity across age groups was observed in all DOMI study sites and sites from the Typhoid Fever Surveillance in Africa Program. 11 , 16
Mortality from typhoid and paratyphoid fevers is difficult to estimate, because cases identified with typhoid fever during surveillance should receive appropriate clinical management and deaths presumed due to typhoid should receive scrutiny to rule out other possible causes. Nonetheless, typhoid and paratyphoid fevers were estimated to be associated with approximately 200,000 deaths in 2000 with absolute number of deaths estimated to be the highest in Africa and South Asia. 5 Figure 2 presents global typhoid and paratyphoid mortality estimates from GBD 2015. Deaths due to paratyphoid fever tend to be lower than those of typhoid fever. 18 However, this difference could be attributed to the incidence of paratyphoid fever being lower than that of typhoid in many settings. The number of paratyphoid fever deaths is estimated to be the greatest in South Asia, where its incidence is also the greatest although far lower than that of typhoid. Before the introduction of antimicrobials, death occurred in as many as 33% of typhoid fever patients in hospital and community settings from developing countries and was seen in upward of 10% of cases in developed countries. 19 The use of antimicrobials initially lowered typhoid fever case fatality below 2% 19 but the emergence of antimicrobial resistant strains in high-burden countries has been a growing concern in recent years.
Strategies for typhoid control and preventive measures.
A range of strategies exist to prevent and control typhoid fever ( Table 1 ). Factors such as crowding, poor sanitation, unsafe water, and unsafe food production and handling processes contribute to Salmonella Typhi and Salmonella Paratyphi transmission. Therefore, measures to interrupt transmission through improvements in sanitation, drinking water, and food production need to be included in comprehensive prevention strategies for enteric fever. 10 In addition to the aforementioned strategies that target risk factors, interventions focused on timely diagnosis and appropriate clinical management can also improve typhoid fever outcomes ( Table 1 ).
Control measures for the management of typhoidal Salmonella
* Vaccines for paratyphoid fever are not available.
The use of vaccines in the control of typhoid fever has been successful as a preventative measure and during outbreak situations in many contexts. In China, during the 1999 typhoid outbreak, the Vi capsular polysaccharide (ViCPS) vaccine showed a protective efficacy of 73% in children previously vaccinated and 71% in children who received the vaccine during the outbreak. 21 In Thailand in 1977 a national typhoid immunization program was implemented in schoolchildren using the heat/phenol-inactivated typhoid vaccine. After the introduction of this program the isolation rate of S. Typhi decreased from 4.6% in 1976 to 0.3% in 1985. 22
Early diagnosis of typhoid and paratyphoid fever.
From a clinician’s perspective typhoid and paratyphoid fever are indistinguishable. Furthermore, many other acute febrile illnesses such as dengue, leptospirosis, and malaria may present a clinical picture similar to that of typhoid fever. Accurate diagnosis requires laboratory confirmation. 23 The development of practical, affordable, and accurate (i.e., both sensitive and specific) diagnostic tools is key to typhoid fever management and control. Typhoid fever can be diagnosed using a number of methods including culture of blood, bone marrow, or stool, and nucleic acid amplification tests (NAAT) for detecting bacterial nucleic acids in appropriate body fluids including blood or bone marrow. 24 Techniques such as NAAT and bone marrow cultures are not, however, feasible in low-income settings, and blood culture or Widal tests are more commonly used.
Not all diagnostic methods perform equally well. Bacterial culture with bone marrow offers the greatest sensitivity at upward of 80%. 23 However, bone marrow aspiration and culture is expensive and invasive and is not commonly used in practice. Consequently, although less sensitive, blood culture remains the practical standard for typhoid fever diagnosis. 23 A recent systematic review of 10 studies examined the sensitivity of blood cultures relative to bone marrow cultures from a sample of 529 positive Salmonella Typhi cases (using blood and bone marrow positivity as a composite measure) and found that bone marrow cultures were positive in 96% of typhoid fever cases whereas blood cultures were positive in 61%. 25 A study by Lee et al. 26 states to accurately detect bloodstream infections such as S. Typhi, two to three 20 mL samples are required for adults. Blood culture sensitivity is the greatest in the initial stages of the infection and, with an adequate sample, has been observed to approach the sensitivity of bone marrow culture. 24 , 26 , 27 Still, relying on blood cultures alone will underestimate the true incidence of typhoid fever. Stool cultures are still used in many endemic regions. However, a positive result may occur in acute disease, convalescent shedding, or chronic carriage. 28 The Widal agglutination test works by measuring antibodies in serum. Although still used in resource-poor settings, the Widal test is inferior to blood culture in terms of both sensitivity and specificity. 28 Other serological detection tests, although sometimes superior to Widal, also lack accuracy for typhoid fever diagnosis. 23
Because sensitivity is < 100%, and has the potential to be influenced by over- or underfill of sample vials and contaminants within samples, case detection with a single-blood culture underestimates typhoid fever incidence. Antimicrobial use, which is a particular concern in high-burden regions, can inhibit bacterial growth and produce false-negative blood cultures, also resulting in underestimating typhoid fever prevalence in febrile patients. Additional diagnostic challenges in low-resource settings include capacity limitations (i.e., shortages in trained staff, diagnostic tools, quality control etc.) that make it difficult to distinguish typhoid from paratyphoid fevers and other causes of acute febrile illness. 29
Treatment with antimicrobial agents.
Effective treatment with antimicrobials was introduced in the 1950s and has been shown to decrease typhoid fever case fatality risk from 30% to 0.5%. 30 However, multidrug-resistant (MDR) typhoidal Salmonella , defined as strains resistant to ampicillin, chloramphenicol, and trimethoprim sulfamethoxazole, emerged in the 1980s. This led to the adoption of fluoroquinolones, such as ciprofloxacin, as the new first-line treatment with extended-spectrum cephalosporins as an alternative. 31 Salmonella strains with decreased susceptibility to ciprofloxacin and cephalosporins have also begun to emerge in high-burden regions, such as South and Southeast Asia. 32 As resistance to standard antimicrobial classes increase, new antimicrobials such as carbapenems, tigecycline, and azithromycin are being assessed as potential treatment options. 33 The transition to new antimicrobials for treatment has caused a drop in MDR-resistant strains of Salmonella Typhi. 34
Three typhoid fever vaccines are currently pre-qualified by the World Health Organization and licensed for use in many countries, the Ty21a oral vaccine, the ViCPS injectable vaccine and more recently the first conjugate vaccine Tybar typhoid conjugate vaccine (TCV), already in use in India and Nepal in babies as young as 6 months of age. 35 , 36 The Ty21a vaccine contains a live, attenuated strain of Salmonella Typhi and demonstrates a protective efficacy between 67% and 80% and the ViCPS vaccine provides a 72% protective efficacy when used in endemic areas and during an outbreak situation. 37 − 39 A trial conducted using the new Tybar TCV vaccine showed the protective vaccine efficacy to be 54.6%. 36 Of the two older vaccines, neither is licensed for use in children less than 2 years of age 35 and while providing protective benefits against typhoid fever neither protects against infection with Salmonella Paratyphi for which no vaccine is currently available.
Despite more information of higher quality available now than ever before, and a range of proven options for prevention, gaps remain in our understanding of typhoid fever burden and the best way to implement prevention strategies in low-resource settings. The findings from our comprehensive exercise to characterize trends in typhoid fever both globally and within endemic countries, summarized in this supplement, will help to fill some of these remaining gaps. The decisions to implement appropriate public health measures and preventive strategies for typhoid fever and other invasive Salmonella infections depend, in part, on the availability of locally improved laboratory diagnostics and relevant data on burden and contextual factors. Accurately estimating the burden of typhoid fever is challenging because data are scarce and derived from varied methods. 1 There is uncertainty around the relative value of investments in health systems and large-scale engineering interventions, such as investments in water and sanitation, food safety measures, public awareness, improved diagnostics, treatment strategies, and immunization programs. The heterogeneity present in water, sanitation, and hygiene infrastructure, sociodemographic determinants, diagnostic test methods, and therapeutic procedures both within and between countries with endemic typhoid fever suggests that enteric fever cannot be eliminated by a single solution in every setting. There is hope regarding the control of enteric fever with the availability of the new Tybar TCV conjugate vaccine used as a complementary tool with the usual public health recommendations on water supply and sanitation.
Author Notes
Financial support: Funding for this study (Grant # OPP1126230, Principal Investigator Zulfiqar A. Bhutta) to the Centre for Global Child Health, Hospital for Sick Children, Toronto, was provided by the Bill & Melinda Gates Foundation ( https://www.gatesfoundation.org/ ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ addresses: Amruta Radhakrishnan and Daina Als, Centre for Global Child Health, The Hospital for Sick Children, Toronto, Canada, E-mails: [email protected] and [email protected] . Eric D. Mintz, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: [email protected] . John A. Crump, Centre for International Health, University of Otago, Dunedin, New Zealand, E-mail: [email protected] . Jefferey Stanaway, Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, E-mail: [email protected] . Robert F. Breiman, Emory University, Atlanta, GA, E-mail: [email protected] . Zulfiqar A. Bhutta, Centre for Global Child Health, The Hospital for Sick Children, Toronto, ON M5G 0A4, Canada, E-mail: [email protected] or Center of Excellence in Women and Child Health, The Aga Khan University, Karachi, Pakistan, E-mail: [email protected] .
American Journal of Tropical Medicine and Hygiene
241 18th Street South, Suite 501
Arlington, VA 22202 USA
© 2022 The American Journal of Tropical Medicine and Hygiene
Access brought to you by:
Powered by: PubFactory
- [66.249.64.20|185.80.151.41]
- 185.80.151.41
Character limit 500 /500

IMAGES
COMMENTS
Introduction Typhoid fever is one of the most common vaccine-preventable diseases in travellers returning from tropical destinations. However, immunity and the immune response to infection are barely understood. Case presentation We report a case of tyhoid fever in a 29-year-old Caucasian, previously healthy woman who did not develop protective immunity or seroconversion of H or O antibodies ...
Blood cultures confirmed XDR S. Typhi. This case highlights three important items: the emergence of the XDR typhoid strain in an unstudied community, the susceptibility of immunocompromised individuals to infectious diseases, and the role health care practitioners can play in controlling its spread regionally and globally.
Case presentation. We report on a 57-year-old Sri-Lankan male affected by typhoid fever, the onset of which was accompanied by oropharyngeal candidiasis. This clinical sign was due to a transient cell-mediated immunity depression (CD4+ cell count was 130 cells/mm 3) probably caused by Salmonella typhi infection. Human immunodeficiency virus ...
n N % Studies n N % Studies Shock,or, hypotension 22 133 16.5 4 7 105 6.7 3 Toxicity 208 427 48.7 5 310 968 32.0 5 GIBleeding 29 316 9.2 8 13 365 3.6 10 GIPerforation 4 142 2.8 2 2 493 0.4 4 Relapse,rates,(%) 36 942 3.8 10 13 524 2.5 8 Case,Fatality,(%) 24 1582 1.5 14 31 2399 1.3 15
Typhoid Fever By: Annabella, Karina, Anthony, and Richard What is Typhoid Fever? What is Typhoid Fever? Salmonella typhi high fevers, weakness, stomach pains, headache, and loss of appetite internal bleeding and death can occur but are rare, very may be hard to treat in developing
A consistent finding of typhoid fever disease burden studies in the last two decades has been the high ... typhoid fever episodes are estimated to occur in the age group 0-4 years; including 29.7% of typhoid fever episodes in the <2 year age group, 9.9% in the <1 year age group, and 2.9% in infants <6 months. ...
Typhoid Fever, also known as Salmonella Typhi, is a bacteria that is caused when humans or animals consume any typ e of food or beverage that is contaminated by the urine or feces of humans or animals.. Typhoid Fever is most common on the East Coast of the United States and almost all of Europe.. The side effects to typhoid fever are deadly and not so deadly .
A new typhoid vaccine composed of the Vi capsular polysaccharide. Arch Intern Med. 1995;155:2293 -2299. 3 Yang HH et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ. 2001;79:625-631. 4 Sur D et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India.
This article is the introduction to a 12-paper supplement on global trends in typhoid fever. The Tackling Typhoid (T2) project was initiated in 2015 to synthesize the existing body of literature on typhoidal salmonellae and study national and regional typhoid fever trends. In addition to a global systematic review, eight case studies were undertaken to examine typhoid and paratyphoid fever ...
Among surgical studies, misclassification of non-typhoid causes of intestinal perforation as TIP are increasingly recognized. 138 We were limited by the use of intra- and postoperative findings in classifying ileal perforations as TIP. We attempted to address this limitation by abstracting and presenting the criteria defining a case of TIP by ...