An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Malaria Diagnosis: A Brief Review
Noppadon tangpukdee, chatnapa duangdee, polrat wilairatana, srivicha krudsood.
- Author information
- Article notes
- Copyright and License information
Corresponding author ( [email protected] )
Corresponding author.
Received 2008 Dec 20; Revised 2009 Apr 1; Accepted 2009 Apr 9; Issue date 2009 Jun.
Malaria is a major cause of death in tropical and sub-tropical countries, killing each year over 1 million people globally; 90% of fatalities occur in African children. Although effective ways to manage malaria now exist, the number of malaria cases is still increasing, due to several factors. In this emergency situation, prompt and effective diagnostic methods are essential for the management and control of malaria. Traditional methods for diagnosing malaria remain problematic; therefore, new technologies have been developed and introduced to overcome the limitations. This review details the currently available diagnostic methods for malaria.
Keywords: Plasmodium , malaria, diagnosis, method
INTRODUCTION
Malaria, sometimes called the "King of Diseases", is caused by protozoan parasites of the genus Plasmodium . The most serious and sometimes fatal type of malaria is caused by Plasmodium falciparum . The other human malaria species, P. vivax , P. ovale , P. malariae , and sometimes P. knowlesi can cause acute, severe illness but mortality rates are low. Malaria is the most important infectious disease in tropical and subtropical regions, and continues to be a major global health problem, with over 40% of the world's population exposed to varying degrees of malaria risk in some 100 countries. It is estimated that over 500 million people suffer from malaria infections annually, resulting in about 1-2 million deaths, of whom 90% are children in sub- Saharan Africa [ 1 ]. The number of malaria cases worldwide seems to be increasing, due to increasing transmission risk in areas where malaria control has declined, the increasing prevalence of drugresistant strains of parasites, and in a relatively few cases, massive increases in international travel and migration [ 2 ]. The need for effective and practical diagnostics for global malaria control is increasing [ 3 ], since effective diagnosis reduces both complications and mortality from malaria. Differentiation of clinical diagnoses from other tropical infections, based on patients' signs and symptoms or physicians' findings, may be difficult. Therefore, confirmatory diagnoses using laboratory technologies are urgently needed. This review discusses on the currently available diagnostic methods for malaria in many settings, and assesses their feasibility in resource-rich and resource-poor settings.
DIAGNOSIS OF MALARIA
Prompt and accurate diagnosis is critical to the effective management of malaria. The global impact of malaria has spurred interest in developing effective diagnostic strategies not only for resource-limited areas where malaria is a substantial burden on society, but also in developed countries, where malaria diagnostic expertise is often lacking [ 4 , 5 ]. Malaria diagnosis involves identifying malaria parasites or antigens/products in patient blood. Although this may seem simple, the diagnostic efficacy is subject to many factors. The different forms of the 5 malaria species; the different stages of erythrocytic schizogony, the endemicity of different species, the interrelation between levels of transmission, population movement, parasitemia, immunity, and signs and symptoms; drug resistance, the problems of recurrent malaria, persisting viable or non-viable parasitemia, and sequestration of the parasites in the deeper tissues, and the use of chemoprophylaxis or even presumptive treatment on the basis of clinical diagnosis, can all influence the identification and interpretation of malaria parasitemia in a diagnostic test.
Malaria is a potential medical emergency and should be treated accordingly. Delays in diagnosis and treatment are leading causes of death in many countries [ 6 ]. Diagnosis can be difficult where malaria is no longer endemic for healthcare providers unfamiliar with the disease. Clinicians may forget to consider malaria among the potential diagnoses for some patients and not order the necessary diagnostic tests. Technicians may be unfamiliar with, or lack experience with, malaria, and fail to detect parasites when examining blood smears under a microscope. In some areas, malaria transmission is so intense that a large proportion of the population is infected but remains asymptomatic, e.g., in Africa. Such carriers have developed sufficient immunity to protect them from malarial illness, but not infection. In such situations, finding malaria parasites in an ill person does not necessarily mean that the illness is caused by the parasites. In many malaria-endemic countries, the lack of resources is a major barrier to reliable and timely diagnosis. Health personnel are undertrained, underequipped, and underpaid. They often face excessive patient loads, and must divide their attention between malaria and other equally severe infectious diseases, such as tuberculosis or HIV/AIDS.
CLINICAL DIAGNOSIS OF MALARIA
A clinical diagnosis of malaria is traditional among medical doctors. This method is least expensive and most widely practiced. Clinical diagnosis is based on the patients' signs and symptoms, and on physical findings at examination. The earliest symptoms of malaria are very nonspecific and variable, and include fever, headache, weakness, myalgia, chills, dizziness, abdominal pain, diarrhea, nausea, vomiting, anorexia, and pruritus [ 7 ]. A clinical diagnosis of malaria is still challenging because of the non-specific nature of the signs and symptoms, which overlap considerably with other common, as well as potentially life-threatening diseases, e.g. common viral or bacterial infections, and other febrile illnesses. The overlapping of malaria symptoms with other tropical diseases impairs diagnostic specificity, which can promote the indiscriminate use of antimalarials and compromise the quality of care for patients with non-malarial fevers in endemic areas [ 8 - 10 ]. The Integrated Management of Children Illness (IMCI) has provided clinical algorithms for managing and diagnosing common childhood illnesses by minimally trained healthcare providers in the developing world having inappropriate equipment for laboratory diagnosis. A widely utilized clinical algorithm for malaria diagnosis, compared with a fully trained pediatrician with access to laboratory support, showed very low specificity (0-9%) but 100% sensitivity in African settings [ 11 , 12 ]. This lack of specificity reveals the perils of distinguishing malaria from other causes of fever in children on clinical grounds alone. Recently, another study showed that use of the IMCI clinical algorithm resulted in 30% over-diagnosis of malaria [ 13 ]. Therefore, the accuracy of malaria diagnosis can be greatly enhanced by combining clinical-and parasite-based findings [ 14 ].
LABORATORY DIAGNOSIS OF MALARIA
Rapid and effective malaria diagnosis not only alleviates suffering, but also decreases community transmission. The nonspecific nature of the clinical signs and symptoms of malaria may result in over-treatment of malaria or non-treatment of other diseases in malaria-endemic areas, and misdiagnosis in non-endemic areas [ 15 ]. In the laboratory, malaria is diagnosed using different techniques, e.g. conventional microscopic diagnosis by staining thin and thick peripheral blood smears [ 16 ], other concentration techniques, e.g. quantitative buffy coat (QBC) method [ 15 ], rapid diagnostic tests e.g., OptiMAL [ 17 , 18 ], ICT [ 19 ], Para-HIT-f [ 10 ], ParaScreen [ 20 ], SD Bioline [ 21 ], Paracheck [ 22 ], and molecular diagnostic methods, such as polymerase chain reaction (PCR) [ 23 , 24 ]. Some advantages and shortcomings of these methods have also been described, related to sensitivity, specificity, accuracy, precision, time consumed, cost-effectiveness, labor intensiveness, the need for skilled microscopists, and the problem of inexperienced technicians.
Microscopic diagnosis using stained thin and thick peripheral blood smears (PBS)
Malaria is conventionally diagnosed by microscopic examination of stained blood films using Giemsa, Wright's, or Field's stains [ 25 ]. This method has changed very little since Laverran's original discovery of the malaria parasite, and improvements in staining techniques by Romanowsky in the late 1,800s. More than a century later, microscopic detection and identification of Plasmodium species in Giemsa-stained thick blood films (for screening the presenting malaria parasite), and thin blood films (for species' confirmation) remains the gold standard for laboratory diagnosis [ 26 ]. Malaria is diagnosed microscopically by staining thick and thin blood films on a glass slide, to visualize malaria parasites. Briefly, the patient's finger is cleaned with 70% ethyl alcohol, allowed to dry and then the side of fingertip is picked with a sharp sterile lancet and two drops of blood are placed on a glass slide. To prepare a thick blood film, a blood spot is stirred in a circular motion with the corner of the slide, taking care not make the preparation too thick, and allowed to dry without fixative. After drying, the spot is stained with diluted Giemsa (1 : 20, vol/vol) for 20 min, and washed by placing the film in buffered water for 3 min. The slide is allowed to air-dry in a vertical position and examination using a light microscope. As they are unfixed, the red cells lyse when a water-based stain is applied. A thin blood film is prepared by immediately placing the smooth edge of a spreader slide in a drop of blood, adjusting the angle between slide and spreader to 45° and then smearing the blood with a swift and steady sweep along the surface. The film is then allowed to air-dry and is fixed with absolute methanol. After drying, the sample is stained with diluted Giemsa (1 : 20, vol/vol) for 20 min and washed by briefly dipping the slide in and out of a jar of buffered water (excessive washing will decolorize the film). The slide is then allowed to air-dry in a vertical position and examined under a light microscope [ 27 ]. The wide acceptance of this technique by laboratories all around the world can be attributed to its simplicity, low cost, its ability to identify the presence of parasites, the infecting species, and assess parasite density-all parameters useful for the management of malaria. Recently, a study showed that conventional malaria microscopic diagnosis at primary healthcare facilities in Tanzania could reduce the prescription of antimalarial drugs, and also appeared to improve the appropriate management of non-malarial fevers [ 16 ]. However, the staining and interpretation processes are labor intensive, time consuming, and require considerable expertise and trained healthcare workers, particularly for identifying species accurately at low parasitemia or in mixed malarial infections. The most important shortcoming of microscopic examination is its relatively low sensitivity, particularly at low parasite levels. Although the expert microscopist can detect up to 5 parasites/µl, the average microscopist detects only 50-100 parasites/µl [ 28 ]. This has probably resulted in underestimating malaria infection rates, especially cases with low parasitemia and asymptomatic malaria. The ability to maintain required levels of in malaria diagnostics expertise is problematic, especially in remote medical centers in countries where the disease is rarely seen [ 29 ]. Microscopy is laborious and ill-suited for high-throughput use, and species determination at low parasite density is still challenging. Therefore, in remote rural settings, e.g. peripheral medical clinics with no electricity and no health-facility resources, microscopy is often unavailable [ 30 ].
QBC technique
The QBC technique was designed to enhance microscopic detection of parasites and simplify malaria diagnosis [ 31 ]. This method involves staining parasite deoxyribonucleic acid (DNA) in micro-hematocrit tubes with fluorescent dyes, e.g. acridine orange, and its subsequent detection by epi-fluorescent microscopy. Briefly, finger-prick blood is collected in a hematocrit tube containing acridine orange and anticoagulant. The tube is centrifuged at 12,000 g for 5 min and immediately examined using an epi-fluorescent microscope [ 27 ]. Parasite nuclei fluoresces bright green, while cytoplasm appears yellow-orange. The QBC technique has been shown to be a rapid and sensitive test for diagnosing malaria in numerous laboratories settings [ 15 , 32 - 35 ]. While it enhances sensitivity for P. falciparum , it reduces sensitivity for non-falciparum species and decreases specificity due to staining of leukocyte DNA [ 36 ]. Recently, it has been shown that acridine orange is the preferred diagnostic method (over light microscopy and immunochromatographic tests) in the context of epidemiologic studies in asymptomatic populations in endemic areas, probably because of increased sensitivity at low parasitemia [ 37 ]. Nowadays, portable fluorescent microscopes using light emitting diode (LED) technology, and pre-prepared glass slides with fluorescent reagent to label parasites, are available commercially [ 38 ]. Although the QBC technique is simple, reliable, and user-friendly, it requires specialized instrumentation, is more costly than conventional light microscopy, and is poor at determining species and numbers of parasites.
Rapid diagnostic tests (RDTs)
Since the World Health Organization (WHO) recognized the urgent need for new, simple, quick, accurate, and cost-effective diagnostic tests for determining the presence of malaria parasites, to overcome the deficiencies of light microscopy, numerous new malaria-diagnostic techniques have been developed [ 39 ]. This, in turn, has led to an increase in the use of RDTs for malaria, which are fast and easy to perform, and do not require electricity or specific equipment [ 40 ]. Currently, 86 malaria RDTs are available from 28 different manufacturers [ 41 ]. Unlike conventional microscopic diagnosis by staining thin and thick peripheral blood smears, and QBC technique, RDTs are all based on the same principle and detect malaria antigen in blood flowing along a membrane containing specific anti-malaria antibodies; they do not require laboratory equipment. Most products target a P. falciparum -specific protein, e.g. histidine-rich protein II (HRP-II) or lactate dehydrogenase (LDH). Some tests detect P. falciparum specific and pan-specific antigens (aldolase or pan-malaria pLDH), and distinguish non- P. falciparum infections from mixed malaria infections. Although most RDT products are suitable for P. falciparum malaria diagnosis, some also claim that they can effectively and rapidly diagnose P. vivax malaria [ 21 , 42 , 43 ]. Recently, a new RDT method has been developed for detecting P. knowlesi [ 44 ]. RDTs provide an opportunity to extend the benefits of parasite-based diagnosis of malaria beyond the confines of light microscopy, with potentially significant advantages in the management of febrile illnesses in remote malaria-endemic areas. RDT performance for diagnosis of malaria has been reported as excellent [ 14 , 19 , 20 , 22 , 45 - 47 ]; however, some reports from remote malaria-endemic areas have shown wide variations in sensitivity [ 36 , 40 , 48 ]. Murray and co-authors recently discussed the reliability of RDTs in an "update on rapid diagnostic testing for malaria" in their excellent paper [ 49 ]. Overall, RDTs appears a highly valuable, rapid malaria-diagnostic tool for healthcare workers; however it must currently be used in conjunction with other methods to confirm the results, characterize infection, and monitor treatment. In malaria-endemic areas where no light microscopy facility exists that may benefit from RDTs, improvements are required for ease of use, sensitivity for non-falciparum infection, stability, and affordability. The WHO is now developing guidelines to ensure lot-to-lot quality control, which is essential for the community's confidence in this new diagnostic tool [ 41 ]. Because the simplicity and reliability of RDTs have been improved for use in rural endemic areas, RDT diagnosis in non-endemic regions is becoming more feasible, which may reduce time-to-treatment for cases of imported malaria [ 30 ].
Serological tests
Diagnosis of malaria using serological methods is usually based on the detection of antibodies against asexual blood stage malaria parasites. Immunofluorescence antibody testing (IFA) has been a reliable serologic test for malaria in recent decades [ 50 ]. Although IFA is time-consuming and subjective, it is highly sensitive and specific [ 51 ]. The literature clearly illustrates the reliability of IFA, so that it was usually regarded as the gold standard for malarial serology testing [ 47 ]. IFA is useful in epidemiological surveys, for screening potential blood donors, and occasionally for providing evidence of recent infection in non-immunes. Until recently, it was a validated method for detecting Plasmodium -specific antibodies in various blood bank units, which was useful for screening prospective blood donors, so avoiding transfusion-transmitted malaria [ 52 , 53 ]. In France, for example, IFA is used as a part of a targeted screening strategy, combined with a donor questionnaire [ 54 ]. The principle of IFA is that, following infection with any Plasmodium species, specific antibodies are produced within 2 wk of initial infection, and persist for 3-6 months after parasite clearance. IFA uses specific antigen or crude antigen prepared on a slide, coated and kept at -30℃ until used, and quantifies both IgG and IgM antibodies in patient serum samples. Titers > 1 : 20 are usually deemed positive, and < 1 : 20 unconfirmed. Titers > 1 : 200 can be classified as recent infections [ 27 ]. In conclusion, IFA is simple and sensitive, but time-consuming. It cannot be automated, which limits the number of sera that can be studied daily. It also requires fluorescence microscopy and trained technicians; readings can be influenced by the level of training of the technician, particularly for serum samples with low antibody titers. Moreover, the lack of IFA reagent standardization makes it impractical for routine use in blood-transfusion centers, and for harmonizing inter-laboratory results.

MOLECULAR DIAGNOSTIC METHODS
As mentioned above, traditional malaria diagnostic methods remain problematic. New laboratory diagnostic techniques that display high sensitivity and high specificity, without subjective variation, are urgently needed in various laboratories. Recent developments in molecular biological technologies, e.g. PCR, loop-mediated isothermal amplification (LAMP), microarray, mass spectrometry (MS), and flow cytometric (FCM) assay techniques, have permitted extensive characterization of the malaria parasite and are generating new strategies for malaria diagnosis.
PCR technique
PCR-based techniques are a recent development in the molecular diagnosis of malaria, and have proven to be one of the most specific and sensitive diagnostic methods, particularly for malaria cases with low parasitemia or mixed infection [ 55 ]. The PCR technique continues to be used extensively to confirm malaria infection, follow-up therapeutic response, and identify drug resistance [ 27 ]. It was found to be more sensitive than QBC and some RDTs [ 56 , 57 ]. Concerning with the gold standard method for malaria diagnosis, PCR has shown higher sensitivity and specificity than conventional microscopic examination of stained peripheral blood smears, and now seems the best method for malaria diagnosis [ 55 ]. PCR can detect as few as 1-5 parasites/µl of blood (≤ 0.0001% of infected red blood cells) compared with around 50-100 parasites/µl of blood by microscopy or RDT. Moreover, PCR can help detect drug-resistant parasites, mixed infections, and may be automated to process large numbers of samples [ 58 , 59 ]. Some modified PCR methods are proving reliable, e.g., nested PCR, real-time PCR, and reverse transcription PCR, and appear to be useful second-line techniques when the 96 Korean J Parasitol. Vol. 47, No. 2: 93-102, June 2009 results of traditional diagnostic methods are unclear for patients presenting with signs and symptoms of malaria; they also allow accurate species determination [ 58 , 60 - 62 ]. Recently, the PCR method has become widely accepted for identifying P. knowlesi infections [ 63 - 65 ]. Although PCR appears to have overcome the two major problems of malaria diagnosis-sensitivity and specificity- the utility of PCR is limited by complex methodologies, high cost, and the need for specially trained technicians. PCR, therefore, is not routinely implemented in developing countries because of the complexity of the testing and the lack of resources to perform these tests adequately and routinely [ 66 ]. Quality control and equipment maintenance are also essential for the PCR technique, so that it may not be suitable for malaria diagnosis in remote rural areas or even in routine clinical diagnostic settings [ 67 ].
LAMP technique
The LAMP technique is claimed to be a simple and inexpensive molecular malaria-diagnostic test that detects the conserved 18S ribosome RNA gene of P. falciparum [ 68 ]. Other studies have shown high sensitivity and specificity, not only for P. falciparum , but also P. vivax , P. ovale and P. malariae [ 69 , 70 ]. These observations suggest that LAMP is more reliable and useful for routine screening for malaria parasites in regions where vector-borne diseases, such as malaria, are endemic. LAMP appears to be easy, sensitive, quick and lower in cost than PCR. However, reagents require cold storage, and further clinical trials are needed to validate the feasibility and clinical utility of LAMP [ 30 ].
Microarrays
Publication of the Plasmodium genome offers many malaria-diagnostic opportunities [ 71 , 72 ]. Microarrays may play an important role in the future diagnosis of infectious diseases [ 73 ]. The principle of the microarrays technique parallels traditional Southern hybridization. Hybridization of labeled targets divided from nucleic acids in the test sample to probes on the array enables the probing of multiple gene targets in a single experiment. Ideally, this technique would be miniaturized and automated for point-of-care diagnostics [ 23 ]. A pan-microbial oligonucleotide microarray has been developed for infectious disease diagnosis and has identified P. falciparum accurately in clinical specimens [ 74 ]. This diagnostic technique, however, is still in the early stages of development [ 30 ].
Flow cytometry has reportedly been used for malaria diagnosis [ 75 - 77 ]. Briefly, the principle of this technique is based on detection of hemozoin, which is produced when the intra-erythrocytic malaria parasites digest host hemoglobin and crystallize the released toxic heme into hemozoin in the acidic food vacuole. Hemozoin within phagocytotes can be detected by depolarization of laser light, as cells pass through a flow-cytometer channel. This method may provide a sensitivity of 49-98%, and a specificity of 82-97%, for malarial diagnosis [ 78 , 79 ], and is potentially useful for diagnosing clinically unsuspected malaria. The disadvantages are its labor intensiveness, the need for trained technicians, costly diagnostic equipment, and that false-positives may occur with other bacterial or viral infections. Therefore, this method should be considered a screening tool for malaria.
Automated blood cell counters (ACC)
An ACC is a practical tool for malaria diagnosis [ 80 ], with 3 reported approaches. The first used a Cell-Dyn® 3500 apparatus to detect malaria pigment (hemozoin) in monocytes, and showed a sensitivity of 95% and specificity of 88%, compared with the gold-standard blood smear [ 81 ]. The second method also used a Cell-Dyn® 3500, and analyzed depolarized laser light (DLL) to detect malaria infection, with an overall sensitivity of 72% and specificity of 96% [ 82 ]. The third technique used a Beckman Coulter ACC to detect increases in activated monocytes by volume, conductivity, and scatter (VCS), with 98% sensitivity and 94% specificity [ 83 ]. Although promising, none of the 3 techniques is routinely available in the clinical laboratory; further studies are required to improve and validate the instrument and its software. The accuracy these methods promise, for detecting malaria parasites, mean ACC could become a valuable and routine malaria-diagnostic laboratory method.
Mass spectrophotometry
A novel method for in vitro detection of malaria parasites, with a sensitivity of 10 parasites/µl of blood, has been reported recently. It comprises a protocol for cleanup of whole blood samples, followed by direct ultraviolet laser desorption mass spectrometry (LDMS). For malaria diagnosis, the principle of LDMS is to identify a specific biomarker in clinical samples. In malaria, heme from hemozoin is the parasite-specific biomarker of interest. LDMS is rapid, high throughput, and automated. Compared with the microscopic method, which requires a skilled microscopist and up to 30-60 min to examine each peripheral blood smear, LDMS can analyze a sample in < 1 min [ 84 ]. However, the remote rural areas without electricity are inhospitable for existing high-tech mass spectrometers. Future improvements in equipment and techniques should make this method more practicable.
Recently, other reliable malaria-diagnostic tests have been developed and introduced, and some tests are commercially available, for example, enzyme linked immunosorbent assay (ELISA)/enzyme immunoassay (EIA) [ 50 , 54 , 85 ], latex agglutination assay [ 86 ], and cultivation of live malaria parasites [ 87 , 88 ]. Post-mortem organ diagnoses, by investigating malaria parasites in tissue autopsy, e.g. liver and spleen [ 89 ], kidney [ 90 ] and brain [ 91 ], have also been described. However, parasite culture, molecular techniques, serology techniques and pathobiological diagnostic techniques, although sometimes useful in research laboratories, are not practical or appropriate for the routine clinical diagnosis of malaria. Table 1 summarizes of modalities and issues for consideration in malaria diagnosis.
Summary of modalities and issues for consideration in malaria diagnosis
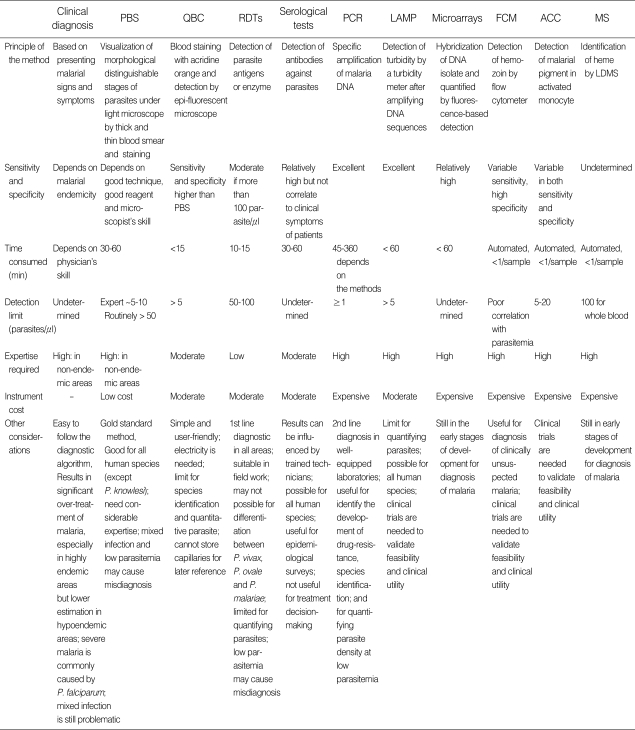
PBS, peripheral blood smears; QBC, quantitative buffy coat; RDTs, rapid diagnostic tests; PCR, polymerase chain reaction; LAMP, loop-mediated isothermal amplification; FCM, flow cytometry; ACC, automated blood cell counter; MS, mass spectrometry; LDMS, laser desorption mass spectrometry.
Conventional microscopic examination of peripheral thick and thin blood smears remains the gold standard for malaria diagnosis. Although this method requires a trained microscopist, and sensitivity and specificity vary compared with recent technical advances, it is inexpensive and reliable. Quick and convenient RDTs are currently implemented in many remote settings, but are costly and need improved quality control. Serological tests are useful for epidemiological surveys, but not suitable for the diagnosis of acute malaria. Molecular-biological techniques are appropriate for research laboratories; they can be used to identify the development of drug-resistance, are useful for species identification, and also for quantifying parasite density with low parasitemia. Finally, the level of malaria endemicity, the urgency of diagnosis, the experience of the physician, the effectiveness of healthcare workers, and budget resources, are all factors influencing the choice of malaria-diagnostic method.
ACKNOWLEDGEMENTS
The authors thank Dr. Kevin C. Kain, of the McLaughlin Center for Molecular Medicine, University of Toronto, Canada, and Dr. Yaowalark Sukthana, Department of Protozoology, Faculty of Tropical Medicine, Mahidol University, for their advice. Thanks to Mr. Paul Adams for editing the English language.
The authors declare no conflict of interest.
- 1. Curing malaria together. [Accessed October 16, 2008];MMV website. Available at: http://www.mmv.org .
- 2. Pasvol G. Management of severe malaria: interventions and controversies. Infect Dis Clin North Am. 2005;19:211–240. doi: 10.1016/j.idc.2004.10.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. World Health Organization. Guidelines for the treatment of malaria. 1st ed. Geneva, Switzerland: WHO; 2006. pp. 133–143. [ Google Scholar ]
- 4. Bell DR, Jorgensen P, Christophel EM, Palmer KL. Malaria risk: estimation of the malaria burden. Nature. 2005;437:E3–E4. doi: 10.1038/nature04179. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJ. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Malaria Facts. [Accessed October 10, 2008];CDC website. Available at: http://www.cdc.gov/malaria/facts.htm .
- 7. Looareesuwan S. Malaria. In: Looareesuwan S, Wilairatana P, editors. Clinical Tropical Medicine. 1st ed. Bangkok, Thailand: Medical Media; 1999. pp. 5–10. [ Google Scholar ]
- 8. Mwangi TW, Mohammed M, Dayo H, Snow RW, Marsh K. Clinical algorithms for malaria diagnosis lack utility among people of different age groups. Trop Med Int Health. 2005;10:530–536. doi: 10.1111/j.1365-3156.2005.01439.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, Whitty CJ. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. McMorrow ML, Masanja MI, Abdulla SM, Kahigwa E, Kachur SP. Challenges in routine implementation and quality control of rapid diagnostic tests for malaria-Rufiji District, Tanzania. Am J Trop Med Hyg. 2008;79:385–390. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Perkins BA, Zucker JR, Otieno J, Jafari HS, Paxton L, Redd SC, Nahlen BL, Schwartz B, Oloo AJ, Olargo C, Gove S, Campbell CC. Evaluation of an algorithm for integrated management of childhood illness in an area of Kenya with high malaria transmission. Bull World Health Organ. 1997;75:33–42. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Weber MW, Mulholland EK, Jaffar S, Troedsson H, Gove S, Greenwood BM. Evaluation of an algorithm for the integrated management of childhood illness in an area with seasonal malaria in the Gambia. Bull World Health Organ. 1997;75:25–32. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Tarimo DS, Minjas JN, Bygbjerg IC. Malaria diagnosis and treatment under the strategy of the integrated management of children illness (IMCI): relevance of laboratory support from the rapid immunochromatographic tests of ICT malaria P.f/P.v and OptiMAL. Ann Trop Med Parasitol. 2001;95:437–444. doi: 10.1080/13648590120068971. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Bhandari PL, Raghuveer CV, Rajeev A, Bhandari PD. Comparative study of peripheral blood smear, quantitative buffy coat and modified centrifuged blood smear in malaria diagnosis. Indian J Pathol Microbiol. 2008;51:108–112. doi: 10.4103/0377-4929.40419. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Ngasala B, Mubi M, Warsame M, Petzold MG, Massele AY, Gustafsson LL, Tomson G, Premji Z, Bjorkman A. Impact of training in clinical and microscopy diagnosis of childhood malaria on anti-malarial drug prescription and health outcome at primary health care level in Tanzania: a randomized controlled trial. Malar J. 2008;7:199. doi: 10.1186/1475-2875-7-199. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Tagbor H, Bruce J, Browne E, Greenwood B, Chandramohan D. Performance of the OptiMAL dipstick in the diagnosis of malaria infection in pregnancy. Ther Clin Risk Manag. 2008;4:631–636. doi: 10.2147/tcrm.s2809. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Zerpa N, Pabón R, Wide A, Gavidia M, Medina M, Cáceres JL, Capaldo J, Baker M, Noya O. Evaluation of the OptiMAL test for diagnosis of malaria in Venezuela. Invest Clin. 2008;49:93–101. [ PubMed ] [ Google Scholar ]
- 19. Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D. Evaluation of two new immunochromatographic assays for diagnosis of malaria. Am J Trop Med Hyg. 2008;79:670–672. [ PubMed ] [ Google Scholar ]
- 20. Endeshaw T, Gebre T, Ngondi J, Graves PM, Shargie EB, Ejigsemahu Y, Ayele B, Yohannes G, Teferi T, Messele A, Zerihun M, Genet A, Mosher AW, Emerson PM, Richards FO. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 2008;7:118. doi: 10.1186/1475-2875-7-118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Lee SW, Jeon K, Jeon BR, Park I. Rapid diagnosis of vivax malaria by the SD Bioline Malaria Antigen test when thrombocytopenia is present. J Clin Microbiol. 2008;46:939–942. doi: 10.1128/JCM.02110-07. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Harvey SA, Jennings L, Chinyama M, Masaninga F, Mulholland K, Bell DR. Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J. 2008;7:160. doi: 10.1186/1475-2875-7-160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Holland CA, Kiechle FL. Point-of-care molecular diagnostic systems-past, present and future. Curr Opin Microbiol. 2005;8:504–509. doi: 10.1016/j.mib.2005.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Vo TK, Bigot P, Gazin P, Sinou V, De Pina JJ, Huynh DC, Fumoux F, Parzy D. Evaluation of a real-time PCR assay for malaria diagnosis in patients from Vietnam and in returned travelers. Trans R Soc Trop Med. 2007;101:422–428. doi: 10.1016/j.trstmh.2006.09.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Warhurst DC, Williams JE. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Bharti AR, Patra KP, Chuquiyauri R, Kosek M, Gilman RH, Llanos-Cuentas A, Vinetz JM. Polymerase chain reaction detection of Plasmodium vivax and Plasmodium falciparum DNA from stored serum samples: implications for retrospective diagnosis of malaria. Am J Trop Med Hyg. 2007;77:444–446. [ PubMed ] [ Google Scholar ]
- 27. Chotivanich K, Silamut K, Day NPJ. Laboratory diagnosis of malaria infection-a short review of methods. Aust J Med Sci. 2006;27:11–15. [ Google Scholar ]
- 28. Payne D. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organ. 1988;66:621–628. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Ohrt C, Purnomo, Sutamihardia MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria prevention trials. J Infect Dis. 2002;186:540–546. doi: 10.1086/341938. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Erdman LK, Kain KC. Molecular diagnostic and surveillance tools for global malaria control. Travel Med Infect Dis. 2008;6:82–99. doi: 10.1016/j.tmaid.2007.10.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Clendennen TE, 3rd, Long GW, Baird KJ. QBC and Giemsa stained thick blood films: diagnostic performance of laboratory technologists. Trans R Soc Trop Med Hyg. 1995;89:183–184. doi: 10.1016/0035-9203(95)90486-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Pornsilapatip J, Namsiripongpun V, Wilde H, Hanvanich M, Chutivongse S. Detection of Plasmodia in acridine orange stained capillary tubes (the QBC system) Southeast Asian J Trop Med Public Health. 1990;21:534–540. [ PubMed ] [ Google Scholar ]
- 33. Salako LA, Akinyanju O, Afolabi BM. Comparison of the standard Giemsa-stained thick blood smear with the Quantitative Buffy Coat Technique in malaria diagnosis in Nigeria. Niger Q J Hosp Med. 1999;9:256–269. [ Google Scholar ]
- 34. Barman D, Mirdha BR, Samantray JC, Kironde F, Kabra SK, Guleria R. Evaluation of quantitative buffy coat (QBC) assay and polymerase chain reaction (PCR) for diagnosis of malaria. J Commun Dis. 2003;35:170–181. [ PubMed ] [ Google Scholar ]
- 35. Adeoye GO, Nga IC. Comparison of Quantitative Buffy Coat technique (QBC) with Giemsa-stained Thick Film (GTF) for diagnosis of malaria. Parasitol Int. 2007;56:308–312. doi: 10.1016/j.parint.2007.06.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR. The reliability of diagnostic techniques in diagnosis and management of malaria in absence of a gold standard. Lancet Infect Dis. 2006;6:582–588. doi: 10.1016/S1473-3099(06)70579-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Partec reagents and accessories. [Accessed November 10, 2008];Cytec website. Available at: http://www.partec.com/preview/cms/front_content.php?idcat=119&idart=201&highlight=malaria+diagnosis .
- 39. World Health Organization. WHO information consultation on recent advances in diagnostic techniques and vaccines for malaria: a rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. Bull World Health Organ. 1996;74:47–54. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol. 2006;4:S7–S20. doi: 10.1038/nrmicro1525. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. List of known commercially available antigen-detecting malaria RDTs. [Accessed November 12, 2008];World Health Organization. Available at: http://www.wpro.who.int/sites/rdt .
- 42. Park TS, Kim JH, Kang CI, Lee BH, Jeon BR, Lee SM, Chang CL, Lee EY, Son HC, Kim HH. Diagnostic usefulness of SD malaria antigen and antibody kits for differential diagnosis of vivax Malaria in patients with fever of unknown origin. Korean J Lab Med. 2006;26:241–245. doi: 10.3343/kjlm.2006.26.4.241. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Kim SH, Nam MH, Roh KH, Park HC, Nam DH, Park GH, Han ET, Klein TA, Lim CS. Evaluation of a rapid diagnostic test specific for Plasmodium vivax. Trop Med Int Health. 2008;13:1495–1500. doi: 10.1111/j.1365-3156.2008.02163.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–1752. doi: 10.3201/eid1411.080840. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Chilton D, Malik AN, Armstrong M, Kettelhut M, Parker-Williams J, Chiodini PL. Use of rapid diagnostic tests for diagnosis of malaria in the UK. J Clin Pathol. 2006;59:862–866. doi: 10.1136/jcp.2005.032904. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. [ PubMed ] [ Google Scholar ]
- 47. Doderer C, Heschung A, Guntz P, Cazenave JP, Hansmann Y, Senegas A, Pfaff AW, Abdelrahman T, Candolfi E. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malar J. 2007;6:19. doi: 10.1186/1475-2875-6-19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Murray CK, Bell D, Gasser RA, Wongsrichanalai C. Rapid diagnostic testing for malaria. Trop Med Int Health. 2003;8:876–883. doi: 10.1046/j.1365-3156.2003.01115.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. She RC, Rawlins ML, Mohl R, Perkins SL, Hill HR, Litwin CM. Comparison of immunofluorescence antibody testing and two enzyme immunoassays in the serologic diagnosis of malaria. J Travel Med. 2007;14:105–111. doi: 10.1111/j.1708-8305.2006.00087.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Sulzer AJ, Wilson M, Hall EC. Indirect fluorescent-antibody tests for parasitic diseases. An evaluation of a thick-smear antigen in the IFA test for malaria antibodies. Am J Trop Med Hyg. 1969;18:199–205. [ PubMed ] [ Google Scholar ]
- 52. Reesing HW. European strategies against the parasite transfusion risk. Transfus Clin Biol. 2005;12:1–4. doi: 10.1016/j.tracli.2004.12.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–1978. doi: 10.1056/NEJM200106283442603. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Oh JS, Kim JS, Lee CH, Nam DH, Kim SH, Park DW, Lee CK, Lim CS, Park GH. Evaluation of a malaria antibody enzyme immunoassay for use in blood screening. Mem Inst Oswaldo Cruz. 2008;103:75–78. doi: 10.1590/s0074-02762008005000008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Morassin B, Fabre R, Berry A, Magnaval JF. One year's experience with the polymerase chain reaction as a routine method for the diagnosis of imported malaria. Am J Trop Med Hyg. 2002;66:503–508. doi: 10.4269/ajtmh.2002.66.503. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Makler MT, Palmer CJ, Ager AL. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–433. doi: 10.1080/00034989859401. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, Jahevitra M, Andriantsoanirina V, Ménard D. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. Am J Trop Med Hyg. 2008;78:217–221. [ PubMed ] [ Google Scholar ]
- 58. Swan H, Sloan L, Muyombwe A, Chavalitshewinkoon-Petmitr P, Krudsood S, Leowattana W, Wilairatana P, Looareesuwan S, Rosenblatt J. Evaluation of a real-time polymerase chain reaction assay for the diagnosis of malaria in patients from Thailand. Am J Trop Med Hyg. 2005;73:850–854. [ PubMed ] [ Google Scholar ]
- 59. Hawkes M, Kain KC. Advance in malaria diagnosis. Expert Rev Anti Infect Ther. 2007;5:1–11. doi: 10.1586/14787210.5.3.485. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Imwong M, Pukrittayakamee S, Pongtavornpinyo W, Nakeesathit S, Nair S, Newton P, Nosten F, Anderson TJ, Dondorp A, Day NP, White NJ. Gene amplification of the multidrug resistance 1 gene of Plasmodium vivax isolates from Thailand, Laos, and Myanmar. Antimicrob Agents Chemother. 2008;52:2657–2659. doi: 10.1128/AAC.01459-07. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Mens PF, Schoone GJ, Kager PA, Schallig HD. Detection and identification of human Plasmodium species with real time quantitative nucleic acid sequence based amplification. Malar J. 2006;5:80. doi: 10.1186/1475-2875-5-80. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Mlambo G, Vasquez Y, LeBlanc R, Sullivan D, Kumar N. A filter paper method for the detection of Plasmodium falciparum gametocytes by reverse transcription polymerase chain reaction. Am J Trop Med Hyg. 2008;78:114–116. [ PubMed ] [ Google Scholar ]
- 63. Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee KS, Singh B. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–813. doi: 10.3201/eid1405.071407. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, Tu TM, Loh JP, Leo YS. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–816. doi: 10.3201/eid1405.070863. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Mens PF, van Amerongen A, Sawa P, Kager PA, Schallig HD. Molecular diagnosis of malaria in the field: development of a novel 1-step nucleic acid lateral flow immunoassay for the detection of all 4 human Plasmodium spp. and its evaluation in Mbita, Kenya. Diagn Microbiol Infect Dis. 2008;61:421–427. doi: 10.1016/j.diagmicrobio.2008.03.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Hanscheid T, Grobusch MP. How useful is PCR in the diagnosis of malaria? Trends Parasitol. 2002;18:395–398. doi: 10.1016/s1471-4922(02)02348-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem. 2006;52:303–306. doi: 10.1373/clinchem.2005.057901. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Han ET, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol. 2007;45:2521–2528. doi: 10.1128/JCM.02117-06. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Aonuma H, Suzuki M, Iseki H, Perera N, Nelson B, Igarashi I, Yagi T, Kanuka H, Fukumoto S. Rapid identification of Plasmodium-carrying mosquitoes using loop-mediated isothermal amplification. Biochem Biophys Res Commun. 2008;376:671–676. doi: 10.1016/j.bbrc.2008.09.061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Crameri A, Marfurt J, Mugittu K, Maire N, Regos A, Coppee JY, Sismeiro O, Burki R, Huber E, Laubscher D, Puijalon O, Genton B, Felger I, Beck HP. Rapid microarray-based method for monitoring of all currently known single-nucleotide polymorphisms associated with parasite resistance to antimalaria drugs. J Clin Microbiol. 2007;45:3685–3691. doi: 10.1128/JCM.01178-07. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Felgner PL. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–4694. doi: 10.1002/pmic.200800194. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Patarakul K. Role of DNA microarray in infectious diseases. Chula Med J. 2008;52:147–153. [ Google Scholar ]
- 74. Palacios G, Quan PL, Jabado OJ, Conlan S, Hirschberg DL, Liu Y, Zhai J, Renwick N, Hui J, Hegyi H, Grolla A, Strong JE, Towner JS, Geisbert TW, Jahrling PB, Büchen-Osmond C, Ellerbrok H, Sanchez-Seco MP, Lussier Y, Formenty P, Nichol MS, Feldmann H, Briese T, Lipkin WI. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg Infect Dis. 2007;13:73–81. doi: 10.3201/eid1301.060837. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Wongchotigul V, Suwanna N, Krudsood S, Chindanond D, Kano S, Hanaoka N, Akai Y, Maekawa Y, Nakayama S, Kojima S, Looareesuwan S. The use of flow cytometry as a diagnostic test for malaria parasites. Southeast Asian J Trop Med Public Health. 2004;35:552–559. [ PubMed ] [ Google Scholar ]
- 76. Shapiro HM, Mandy F. Cytometry in malaria: moving beyond Giemsa. Cytometry A. 2007;71:643–645. doi: 10.1002/cyto.a.20453. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Izumiyama S, Omura M, Takasaki T, Ohmae H, Asahi H. Plasmodium falciparum: development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Exp Parasitol. 2009;121:144–150. doi: 10.1016/j.exppara.2008.10.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Grobusch MP, Hänscheid T, Krämer B, Neukammer J, May J, Seybold J, Kun JF, Suttorp N. Sensitivity of hemozoin detection by automated flow cytometry in non- and semi-immune malaria patients. Cytometry B Clin Cytom. 2003;55:46–51. doi: 10.1002/cyto.b.10039. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Padial MM, Subirats M, Puente S, Lago M, Crespo S, Palacios G, Baquero M. Sensitivity of laser light depolarization analysis for detection of malaria in blood samples. J Med Microbiol. 2005;54:449–452. doi: 10.1099/jmm.0.45650-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. de Langen AJ, van Dillen J, de Witte P, Mucheto S, Nagelkerke N, Kager P. Automated detection of malaria pigment: feasibility for malaria diagnosing in an area with seasonal malaria in northern Namibia. Trop Med Int Health. 2006;11:809–816. doi: 10.1111/j.1365-3156.2006.01634.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Hanscheid T, Melo-Cristino J, Pinto BG. Automated detection of malaria pigment in white blood cells for the diagnosis of malaria in Portugal. Am J Trop Med Hyg. 2001;64:290–292. doi: 10.4269/ajtmh.2001.64.290. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Mendelow BV, Lyons C, Nhlangothi P, Tana M, Munster M, Wypkema E, Liebowitz L, Marshall L, Scott S, Coetzer TL. Automated malaria detection by depolarization of laser light. Br J Haematol. 1999;104:499–503. doi: 10.1046/j.1365-2141.1999.01199.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Briggs C, Costa AD, Freeman Lyn, Aucamp I, Ngubeni B, Machin SJ. Development of an automated malaria discriminant factor using VCS technology. Am J Clin Pathol. 2006;126:691–698. doi: 10.1309/0PL3-C674-M39D-6GEN. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Scholl PF, Kongkasuriyachai D, Demirev PA, Feldman AB, Lin JS, Sullivan DJ, Jr, Kumar N. Rapid detection of malaria infection in vivo by laser desorption mass spectrometry. Am J Trop Med Hyg. 2004;71:546–551. [ PubMed ] [ Google Scholar ]
- 85. Park JW, Yoo SB, Oh JH, Yeom JS, Lee YH, Bahk YY, Kim YS, Lim KJ. Diagnosis of vivax malaria using an IgM capture ELISA is a sensitive method, even for low levels of parasitemia. Parasitol Res. 2008;103:625–631. doi: 10.1007/s00436-008-1023-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Polpanich D, Tangboriboonrat P, Elaissari A, Udomsangpetch R. Detection of malaria infection via latex agglutination assay. Anal Chem. 2007;79:4690–4695. doi: 10.1021/ac070502w. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Chotivanich K, Silamut K, Udomsangpetch R, Stepniewska KA, Pukrittayakamee S, Looareesuwan S, White NJ. Ex-vivo short-term culture and developmental assessment of Plasmodium vivax. Trans R Soc Trop Med Hyg. 2001;95:677–680. doi: 10.1016/s0035-9203(01)90113-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Udomsangpetch R, Kaneko O, Chotivanich K, Sattabongkot J. Cultivation of Plasmodium vivax. Trends Parasitol. 2008;24:85–88. doi: 10.1016/j.pt.2007.09.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Prommano O, Chaisri U, Turner GD, Wilairatana P, Ferguson DJ, Viriyavejakul P, White NJ, Pongponratn E. A quantitative ultrastructural study of the liver and the spleen in fatal falciparum malaria. Southeast Asian J Trop Med Public Health. 2005;36:1359–1370. [ PubMed ] [ Google Scholar ]
- 90. Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ, Ferguson DJ, Pongponratn E. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health. 2007;12:1037–1050. doi: 10.1111/j.1365-3156.2007.01881.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Sachanonta N, Medana IM, Roberts R, Jones M, Day NP, White NJ, Ferguson DJ, Turner GD, Pongponratn E. Host vascular endothelial growth factor is tropic for Plasmodium falciparum-infected red blood cells. Asian Pac J Allergy Immunol. 2008;26:37–45. [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (125.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO