- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information

Bachelor of education (90102)
University of south africa, recommended for you, students also viewed.
- TPS3704 Ass50
- 7. Weekly TEST- Ethics & Professionalism
- Prescribed SBA experiment (series circuits)
- Isi Xhosa FAL Grade 11 Term 1 Week 4 2021
- Lsp1501 assignment 13
- Tps3703 ass02 - Excellent
Related documents
- TPN3703 - Portfolio
- TPN2602 Portfolio
- Minor Changes in TMS3722 Study Guide
- 4 5893438374202574484
- Cic2601 assignment 3
- Share BTE 2601 Assignment 3
Preview text
Grade 12 prescribed experiment 2: acid-base reactions, total mark: 50, titration of oxalic acid against sodium hydroxide to determine the concentration of the sodium, in this investigation you will prepare an acidic solution accurately and thus you will know its exact, concentration. you will then react this acid with a base of an unknown concentration to determine, the concentration of the base., what you will need:, erlenmeyer flasks, burette clamp, medicine dropper, retort stand, white tile /paper, measuring cylinders, oxalic acid, sodium hydroxide, phenolphthalein as indicator, pipette with sucker, what to do:, 1. prepare a standard solution of oxalic acid which has a concentration of approximately, 2. now prepare a sodium hydroxide solution by dissolving approximately 2g of dry, sodium hydroxide in 500ml of water., 3. add two drops of the indicator solution., 4. place the burette in the clamp., 5. using the funnel, fill the burette to above zero mark with the acid solution., 6. then, holding the beaker, with which you used to pour the acid, beneath the burette,, gradually open the tap., 7. allow the level of the base to come down to exactly zero (reading from the bottom of, the meniscus)., 8. pipette using the sucker exactly 25ml of oxalic acid solution in a volumetric flask., 9. add a few drops of phenolphthalein to the acid., 10. hold the conical flask beneath the burette with your right hand and gradually open, the tap with your left., 11. swirl the conical flak continuously and watch it closely for the first sign of a colour, 12. as you see that you are approaching the point of neutralization, close the tap slightly, so that you are adding drop by drop., 13. when the colour changes completely the titration is finished., 14. close the tap and read from the burette how much acid was used., 15. repeat this procedure at least twice so that you have three readings for the volume, of naoh (of unknown concentration) required to neutralize exactly 25ml of oxalic, acid (of known concentration)., 16. take an average of these three and use it to calculate the concentration of the naoh., 17. now calculate the concentration of the sodium hydroxide solution., 18. make a neat labeled sketch to represent the apparatus, 19. now write a report using the format learnt in class., 1. what is the appropriate concentration of naoh (2g in 500ml of water), 2. calculate the theoretical concentration of naoh from the actual mass of naoh you, 3. how does your theoretical value for naoh concentration (from the actual mass you, measured) differ from the actual concentration you calculated (from the titration, procedure) can you think of some reasons why your values may differ, (b) calculate the concentration of the unknown solution using the equation, nbcava = nacbvb, ___________________________________________________________________________, ________________________________________________________________________ (7), safety audits on:, oxalic acid √ √, fire combustible. gives off.
irritating or toxic fumes (or gases) in a fire.
NO open flames. Powder, alcohol-resistant foam, water spray, carbon dioxide.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with water.
EXPOSURE AVOID ALL CONTACT! IN ALL CASES CONSULT A
Inhalation sore throat. cough. burning.
sensation. Shortness of breath. Laboured breathing. Symptoms may be delayed (see Notes).
Local exhaust or breathing protection.
Fresh air, rest. Half-upright position. Artificial respiration if indicated. Refer for medical attention.
Skin Redness. Skin burns. Pain.
Protective clothing. First rinse with plenty of water, then remove contaminated clothes and rinse again. Refer for medical attention.
Eyes Redness. Pain. Loss of vision.
Severe deep burns.
Face shield, or eye protection in combination with breathing protection if powder.
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then take to a doctor.

Ingestion Sore throat. Burning
sensation. Abdominal pain. Vomiting. Drowsiness. Shock
Do not eat, drink, or smoke during work. Wash hands before eating.
Rinse mouth. Rest. Refer for medical attention.
or collapse. Convulsions.
Not combustible. Contact with moisture or water may generate sufficient heat to ignite combustible substances.
In case of fire in the surroundings: use appropriate extinguishing media.
AVOID ALL CONTACT! IN ALL CASES CONSULT A
Corrosive. Burning sensation. Sore throat. Cough. Laboured breathing. Shortness of breath. Symptoms may be delayed (see Notes).
Fresh air, rest. Half-upright position. Artificial respiration may be needed. Refer for medical attention.
Corrosive. Redness. Pain. Serious skin burns. Blisters.
Protective gloves. Protective clothing.
Remove contaminated clothes. Rinse skin with plenty of water or shower. Refer for medical attention.
Corrosive. Redness. Pain. Blurred vision. Severe deep burns.
Face shield or eye protection in combination with breathing protection if powder.
Corrosive. Burning sensation. Abdominal pain. Shock or collapse.
Do not eat, drink, or smoke during work.
Rinse mouth. Do NOT induce vomiting. Give plenty of water to drink. Refer for medical attention.
SODIUM HYDROXIDE (NaOH) √ √
Appropriate sketch, n bc av a = n ac bv b, 1x1x 0 = 2 x cb x 0., c b = 0 mol/dm, suggestions on how to improve:, you can let them write an equation before they perform the experiment, don’t ask questions like ‘is it a weak or strong acid: because there is 50%, chance of guessing, pour the oxalic acid in the burette & naoh in the erlenmeyer flask because, cleaning the naoh from burette is difficult & burette might break, you can let the learners prepare the standard solution, completion of a table could have been assessed, e. indicating units in the, include everyday application of e. the oxalic acid (action of the antacid)., list precautions just before the method is given, suggestion: don’t forget: structure the questions in such a way that, for marking,, you use 20% rubric & 80% memorandum, nb: in a practical investigation: you use scientific method. in an experiment you, don’t use a scientific method coz u r verifying a hypothesis., how section 1 (caps) can feature in the experiment, refer to caps page 5 d, bullet 1-, identify and solve problems and make decisions using critical and creative thinking;, work effectively as individuals and with others as members of a team;, organise and manage themselves and their activities responsibly and effectively;, collect, analyse, organise and critically evaluate information;, communicate effectively using visual, symbolic and/or language skills in various.
- Multiple Choice
Course : Bachelor of education (90102)
University : university of south africa.

- Discover more from: Bachelor of education 90102 University of South Africa 999+ Documents Go to course
- More from: Bachelor of education 90102 University of South Africa 999+ Documents Go to course
- For educators
- English (US)
- English (India)
- English (UK)
- Greek Alphabet
This problem has been solved!
You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Question: Experiment #12: Acid-Base Titration NAME: Section: Date: DATA & LAB REPORT: Part A: Determining Molarity of NaOH Solution *Show ALL Calculation Setups Trial 3 Mass of KHP Final Buret Reading Initial Buret Reading Trial 1 0.58589 31.10mL 0.73 mL Trial 2 0.95109 44.10 mL 0.00 mL Volume of NaOH Used 30.37ml 44.10mL 1. Calculate moles of KHP used in each trial.
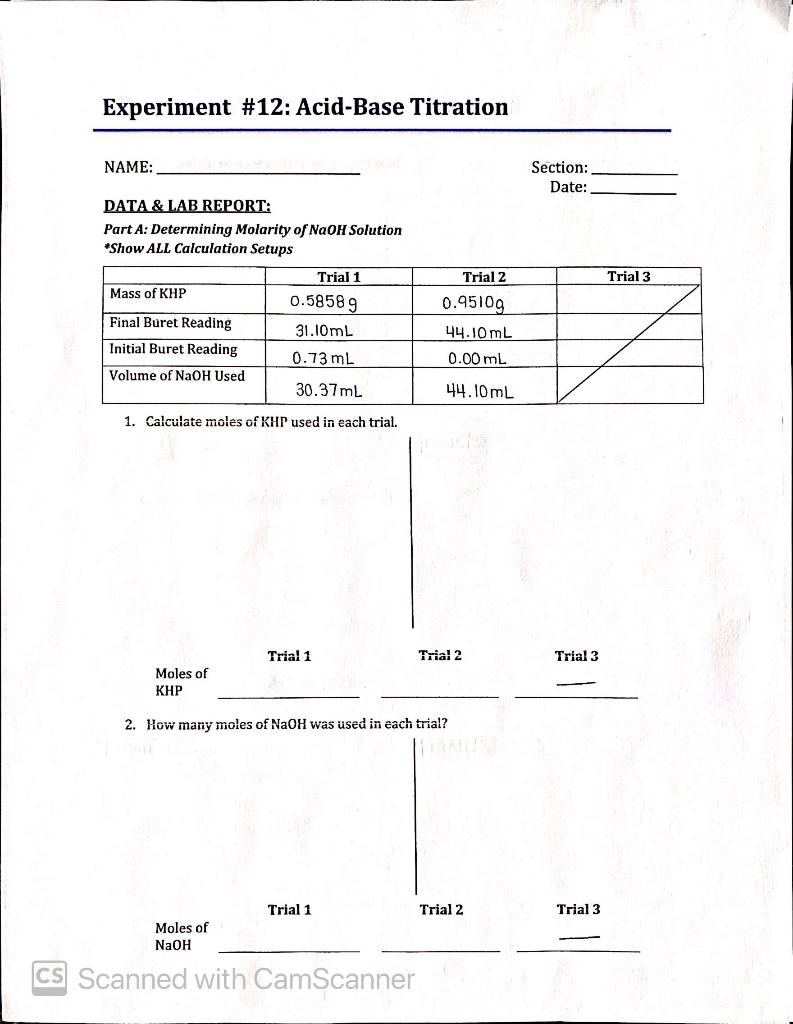
Use significant figures!
This AI-generated tip is based on Chegg's full solution. Sign up to see more!
Convert the mass of KHP in Trial 1 to moles using the formula: .
Conversion of grams to moles: Moles = Mass of the compound (g)/Mass of 1 mole(g/Mole) Mass of 1 mole = Molecular weight of the compound Molecular Weight of KHP(Potassium hydrogen phthalate) = 204.222 grams/mole Mass of KPH in trial 1= 0.5858 g Calcul …

Not the question you’re looking for?
Post any question and get expert help quickly.

IMAGES
VIDEO
COMMENTS
Experiment 12 - Acid/Base Titrations Discussion This experiment demonstrates an analytical technique known as titration, where a solution is delivered from a buret until it completely consumes another solution in a flask. Consider the following: Acid-base titrations are an example of volumetric analysis, a
Experiment 12: Acid/Base Titration I. INTRODUCTION. The object of an acid-base titration is usually to determine the point at which exact neutralization of the acid by the base occurs. At the true neutralization point (known as the equivalence point) an equivalency between the acid and the base has been achieved. In this experiment, we will be ...
Experiment #10/11:Part 1 Acid Base Titration. Abstract: The purpose of this experiment is to observe the titration of hydrochloric acid, a strong acid with sodium hydroxide, a strong base and acetic acid, a weak acid with sodium hydroxide, a strong base. ... 69 sec 104 sec 7 2 12. CH 3 COOH. NaOH; Weak Acid Strong Base. 69 sec 99 sec 8 2 11 ...
titration, calculate the molarity of the NaOH solution. 12. Wash and rinse out your Erlenmeyer flask, top-off the buret with more NaOH solution and repeat the titration process two more times. Part II: Analysis of vinegar (MICROSCALE TITRATION) Vinegar is a dilute solution of acetic acid in water. Using titration techniques the
GRADE 12 PRESCRIBED EXPERIMENT 2: ACID-BASE REACTIONS WORK SHEET TOTAL MARK: 50 ACTIVITY Titration of oxalic acid against sodium hydroxide to determine the concentration of the sodium hydroxide. In this investigation you will prepare an acidic solution accurately and thus you will know its exact concentration.
Experiment #12: Acid-Base Titration NAME: Section: Date: DATA & LAB REPORT: Part A: Determining Molarity of NaOH Solution *Show ALL Calculation Setups Trial 3 Mass of KHP Final Buret Reading Initial Buret Reading Trial 1 0.58589 31.10mL 0.73 mL Trial 2 0.95109 44.10 mL 0.00 mL Volume of NaOH Used 30.37ml 44.10mL 1. Calculate moles of KHP used ...
Experiment 11 - Acid-Base Titration Introduction A titration is an experimental technique for determining the molarity of a solution by reaction with something else. Recall that molarity of a solution is defined as moles of solute per liter of solution, so a 1 M ("one molar") solution has 1 mole of solute in 1 liter of solution.
Experiment 11 - Acid-Base Titration Introduction A titration is an experimental technique for determining the molarity of a substance (the analyte) in solution by reacting it with another substance (the titrant) for which you can keep track of the amount, or volume, of titrant reacted. Recall that molarity of a solution is defined as
Strong acid - Strong base Table 1: Common Indicators and Their Colours In the above example, pH of the hydrochloric acid will change with increasing amount of sodium hydroxide added. At the end-point the solution will become neutral with pH of 7. This is only true for strong acid-strong base titration. For strong acid-weak base, weak acid ...
Grade 12 Chemistry Practical: Acid-base titration. ... Explain the following procedures in this experiment: 4.1 Why is the burette washed in sodium hydroxide solution before starting the titration? (2) 4.2 There is an instruction to wash the drops of sodium hydroxide off the inside surfaces of the